International bi-monthly journal of cell signaling, tissue protection, and translational research.
Preconditioning with CpG-ODN1826 reduces ischemic brain injury in young male mice: a replication study
Kunjan R. Dave1,2,3, Isabel Saul1,2, Ami P. Raval1,2,3, Miguel A. Perez-Pinzon1,2,3
Author Affiliations


- 1Peritz Scheinberg Cerebral Vascular Disease Research Laboratories, University of Miami School of Medicine, Miami, FL, USA
- 2Department of Neurology, University of Miami School of Medicine, Miami, FL, USA
- 3Neuroscience Program, University of Miami School of Medicine, Miami, FL, USA
Abstract
Earlier studies established that ischemic tolerance can be induced in the brain using various strategies. An earlier study demonstrated that preconditioning with the toll-like receptor 9 ligand, CpG oligodeoxynucleotides (ODN), protects the brain against ischemic damage. To increase the potential translational value of the previous study, the goal of the present study was to replicate this earlier finding in a different animal cohort at a different site. In addition to these replication studies, following the Stroke Treatment Academic Industry Roundtable (STAIR) guidelines, we also conducted studies to evaluate the protective effect of CpG-ODN 1826 preconditioning on cerebral ischemic damage in ovariectomized (Ovx) female animals. Young male and female mice were treated with CpG-ODN 1826 or control ligand 3 days prior to the induction of transient (60 min) cerebral ischemia using a middle cerebral artery occlusion (MCAO) model. Infarct size was evaluated at ~24 h post-MCAO. We were able to replicate earlier findings that preconditioning with a low dose (20 µg/mouse) of CpG-ODN 1826 was able to lower cerebral ischemic damage in young male mice. However, we did not see any protective effect of low dose CpG-ODN 1826 preconditioning against cerebral ischemic damage in young Ovx female mice. Our study independently confirms the protective effect of CpG-ODN 1826 in inducing cerebral ischemia tolerance in male but not in Ovx female mice. Our study also demonstrates the feasibility of conducting such replication studies in rodent models of transient stroke.
Keywords: Conditioning, tolerance, stroke, Toll-like receptors, replication, validation, neuroprotection.
Abstract
Earlier studies established that ischemic tolerance can be induced in the brain using various strategies. An earlier study demonstrated that preconditioning with the toll-like receptor 9 ligand, CpG oligodeoxynucleotides (ODN), protects the brain against ischemic damage. To increase the potential translational value of the previous study, the goal of the present study was to replicate this earlier finding in a different animal cohort at a different site. In addition to these replication studies, following the Stroke Treatment Academic Industry Roundtable (STAIR) guidelines, we also conducted studies to evaluate the protective effect of CpG-ODN 1826 preconditioning on cerebral ischemic damage in ovariectomized (Ovx) female animals. Young male and female mice were treated with CpG-ODN 1826 or control ligand 3 days prior to the induction of transient (60 min) cerebral ischemia using a middle cerebral artery occlusion (MCAO) model. Infarct size was evaluated at ~24 h post-MCAO. We were able to replicate earlier findings that preconditioning with a low dose (20 µg/mouse) of CpG-ODN 1826 was able to lower cerebral ischemic damage in young male mice. However, we did not see any protective effect of low dose CpG-ODN 1826 preconditioning against cerebral ischemic damage in young Ovx female mice. Our study independently confirms the protective effect of CpG-ODN 1826 in inducing cerebral ischemia tolerance in male but not in Ovx female mice. Our study also demonstrates the feasibility of conducting such replication studies in rodent models of transient stroke.
Keywords: Conditioning, tolerance, stroke, Toll-like receptors, replication, validation, neuroprotection.
Introduction
Preconditioning paradigms, such as ischemic or pharmacological preconditioning, activate neuroprotective pathways to protect brain against cerebral ischemic injury. The cerebral ischemic tolerance potential of several chemicals, such as inhibitors of the mitochondrial electron transport chain (Riepe et al., 1997; Sugino et al., 1999), lipopolysaccharide (Tasaki et al., 1997; Puisieux et al., 2000; Garcia-Bonilla et al., 2018), adenosine (Perez-Pinzon et al., 1996; Blondeau et al., 2000; Reshef et al., 2000; Hiraide et al., 2001), anesthetics (Kapinya et al., 2002), thrombin (Bao et al., 2018), and resveratrol (Raval et al., 2006; Della-Morte et al., 2009; Koronowski et al., 2015; Narayanan et al., 2015; Koronowski et al., 2017) has been established. An earlier study also demonstrated that pretreatment with unmethylated CpG oligodeoxynucleotides (ODNs), a ligand for Toll-like receptor 9 (TLR9), also induces protection against ischemic brain damage (Stevens et al., 2008). This earlier study also concluded that prophylactic treatment of ‘at risk’ patients with CpG ODNs may offer great translational potential. Another study from the same group also demonstrated the potential of CpG ODNs in inducing pharmacological preconditioning against cerebrovascular ischemic injury in a nonhuman primate model of stroke using the rhesus macaque (Bahjat et al., 2011).
A recommendation of the Stroke Therapy Academic industry Roundtable (STAIR) was for preclinical results from one laboratory to be replicated in an independent laboratory before testing in large, time-consuming, and expensive clinical trials (Stroke Therapy Academic Industry, 1999). This recommendation is further endorsed by a recent NINDS-sponsored workshop “Translational Stroke Research: Vision and Opportunities” (Bosetti et al., 2017). In accordance with this, the goal of this study was to replicate an earlier finding of CpG-ODN 1826 preconditioning-induced neuroprotection in young healthy male mice in our laboratory (Stevens et al., 2008). In addition, we also expanded the study to address an additional STAIR recommendation to test the efficacy of this preconditioning agent in female mice.
Methods
Animals and induction of focal ischemia: middle cerebral artery occlusion (MCAO)
A team from the parent laboratory trained study staff of the partner laboratory prior to beginning this study. The partner laboratory solely performed studies described in this manuscript.
Young male and ovariectomized (Ovx) female mice were purchased from Jackson Laboratories (Bar Harbor, ME). Both male and female mice were 8 - 12 weeks old at the time of the experiment. All experimental procedures were carried out as per the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health and in accordance with the protocols approved by the Animal Care and Use Committee of the University of Miami.
Animals were anesthetized with 3% Isoflurane in a 30% O2/70% N2O mixture and maintained under anesthesia using 1 – 2% isoflurane during the surgery. Rectal and temporalis muscle temperatures were monitored and maintained at 37.0 ± 0.5° C using heating pads during induction of cerebral ischemia. MCAO was induced as previously described in mice employing a silicone-coated 7-0 monofilament nylon surgical suture (Doccol, Sharon, MA) (Stevens et al., 2008). In brief, under an operating microscope, the right common carotid artery (CCA) was exposed through a midline neck incision, the internal carotid artery (ICA) was isolated, the pterygopalatine artery was ligated, a 5-0 silk suture was tied loosely around the mobilized external carotid artery (ECA) stump, and a silicone-coated 7-0 monofilament nylon surgical suture was inserted via the proximal ECA into the ICA, effectively occluding the middle cerebral artery (MCA). After 60 min of MCA occlusion the intraluminal suture was removed, the neck incision was closed with nylon suture, and animals were then returned to their cages upon awakening from anesthesia. Cerebral blood flow (CBF) was monitored by placing a laser Doppler probe (Perimed System, Stockholm, Sweden) on the skull on the MCA occlusion side before, during, and after cerebral ischemia. At the end of ~24h reperfusion period, mice were anesthetized with isoflurane, perfused with ice-cold saline containing 2 U/ml heparin, brains were quickly removed from the cranium, and coronal brain sections were processed for 2,3,5-triphenyltetrazolium chloride (TTC) staining. Infarct volume was determined on TTC-stained coronal brain sections using the “indirect” method to minimize error introduced from edema (Stevens et al., 2008).
CpG ODN 1826 preconditioning
Animals were treated with an intraperitoneal injection (in a volume of 200 µl) of saline, ODN 1826 control (has the same sequence as 1826 but the CpG dinucelotides have been replaced by GpC dinucelotides) (20 and 40 µg/mouse), or ODN 1826 drug (a mouse-specific phosphothioate CpG-ODN ligand for TLR9) (20 and 40 µg/mouse) 3 days prior to the induction of MCAO (Stevens et al., 2008). The CpG ODN 1826 control and CpG ODN 1826 drug were purchased from InvivoGen (San Diego, CA) and were dissolved in saline.
Statistical analysis
Induction of stroke and analysis of infarct volume were performed by an investigator blinded to the experimental conditions. Animals that had more than an 80% drop in CBF during MCAO and survived 1 day post-MCAO were included in the final analysis. Mean differences were analyzed using one-way ANOVA with Bonferroni's post hoc test for multiple group comparisons. Statistical comparison between two groups was performed using Student's t test. Data are presented as mean ± standard error of the mean (SEM).
Results
Replication studies in male mice
In the first set of experiments, we examined the effect of CpG ODN 1826 preconditioning on cerebral ischemic damage in an in vivo model of transient stroke (i.e. mouse model of transient focal cerebral ischemia) (Stevens et al., 2008). Young male mice were treated with saline, ODN 1826 control (20 and 40 µg/mouse), or ODN 1826 drug (20 and 40 µg/mouse) 3 days prior to the induction of MCAO. The duration of MCAO was set at 60 min because during the initial experiments the technician who performed these experiments obtained an infarct volume lower than 50% (47 ± 3%, n = 6), similar to the study that we aimed to replicate (Stevens et al., 2008).
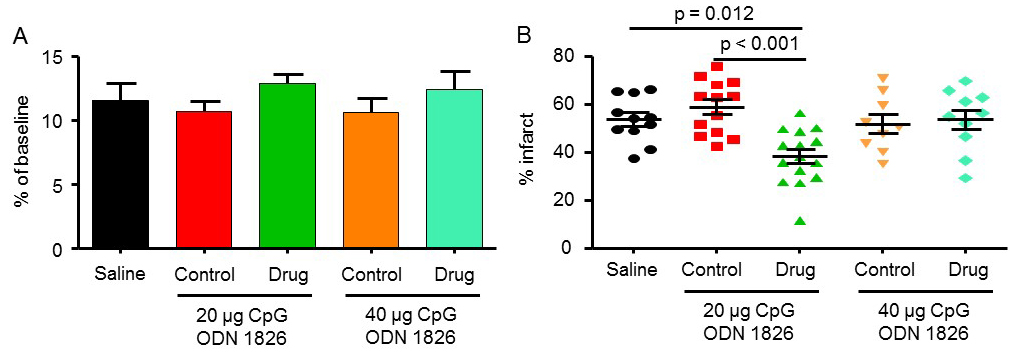
The mean drop in CBF during MCAO for saline, 20 µg ODN 1826 control, 20 µg ODN 1826, 40 µg ODN 1826 control, and 40 µg ODN 1826 was 12 ± 1, 11 ± 1, 13 ± 1, 11 ± 1, and 12 ± 1%, respectively (Figure 1A). The infarct volume in the saline-treated group was 54 ± 3% (n = 11) (Figure 1B). Infarct volumes in the 20 µg and 40 µg ODN 1826 control groups were 59 ± 3% (n = 13) and 52 ± 4% (n = 9), respectively. Infarct volumes in 20 µg and 40 µg ODN 1826 drug groups were 38 ± 3% (n = 15) and 54 ± 4% (n = 10), respectively. The infarct volume was significantly lower in the 20 µg ODN 1826-treated group by 29 and 35% as compared to the saline and 20 µg ODN 1826 control group, respectively. Our results demonstrate that preconditioning of young male mice with low dose (20 µg/mouse) of ODN 1826 protects the brain against cerebral ischemic damage. These results also confirmed the findings of the previously reported study.
In a new window | Download PPT
Figure 1: The effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia. Groups included 1) saline (n = 11), 20 µg ODN 1826 control (n = 13), 20 µg ODN 1826 (n = 15), 40 µg ODN 1826 control (n = 9), and 40 µg ODN 1826-treated (n = 10) young male mice. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
Further confirmation of the protective effect of CpG-ODN1826 preconditioning in a larger number of young male mice
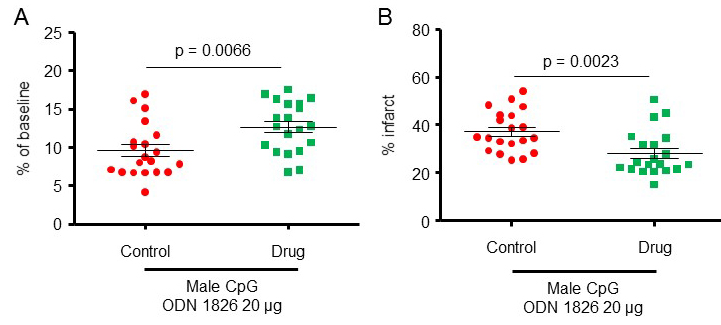
To further confirm a protective effect of CpG-ODN1826 preconditioning, we increased the number of mice/group to 20 for the 20 µg treatment dose for which we observed protective effects. Two experimental groups were included: 1) young male mice treated with 20 µg ODN 1826 control and 2) young male mice treated with 20 µg ODN 1826 drug. Data presented in this section also include data presented for male mice in Figures 1A-B. The CBF during MCAO for male mice treated with 20 µg ODN 1826 control and male mice treated with 20 µg ODN 1826 drug, dropped to 9.6 ± 0.8 (n = 20), and 12.7 ± 0.7% (n = 20) of baseline CBF, respectively (Figure 2A). The infarct volumes in male mice treated with 20 µg ODN 1826 control and male mice treated with 20 µg ODN 1826 drug were 37 ± 2% (n = 20) and 28 ± 2% (n = 20), respectively (Figure 2B). Our results demonstrate that preconditioning of young male mice with a low dose (20 µg) of ODN 1826 drug protects the brain against cerebral ischemic damage even in a larger sample size.
In a new window | Download PPT
Figure 2: The protective effect of CpG-ODN1826 preconditioning in a larger number of young male mice (n = 20/group). The effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia with an increased sample size. Groups included 1) 20 µg ODN 1826 control (n = 20) and 20 µg ODN 1826-treated (n = 20) young male mice. Data presented in this figure also include data presented from male mice in Figures 1A-B. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
The protective effect of CpG-ODN1826 preconditioning in young male mice is not due to a lower drop in CBF during ischemia
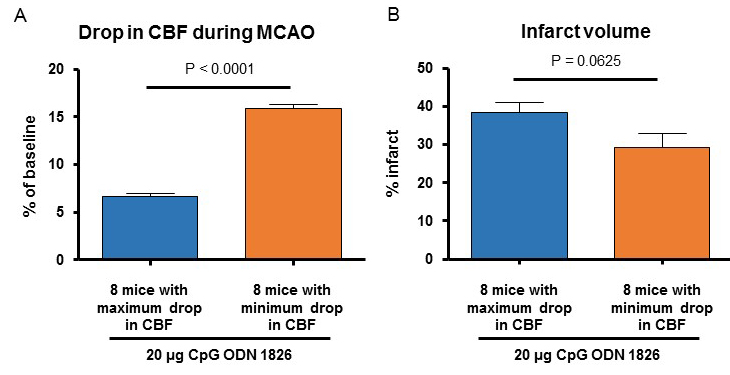
Because we observed a significantly lower CBF drop during MCAO in the CpG ODN 1826 drug-preconditioned young male mice, we performed an additional analysis comparing two sub-populations of the drug-treated group. CBF during MCAO in 8 male mice belonging to the 20 µg ODN 1826 drug-treated group where we observed a maximum drop in CBF and 8 male mice belonging to the 20 µg ODN 1826 drug-treated group where we observed a minimum drop in CBF was 6.6 ± 0.4 (n = 8), and 15.9 ± 0.4% (n = 8) of baseline CBF, respectively (Figure 3A). The infarct volumes in 8 male mice belonging to 20 µg ODN 1826 drug-treated group where we observed a maximum drop in CBF and 8 male mice belonging to 20 µg ODN 1826 drug-treated group where we observed minimum drop in CBF were 38 ± 3 (n = 8), and 29 ± 4% (n = 8) of baseline CBF, respectively (Figure 3B). The difference in infarct volume was not significantly different between the two sub-populations. Our results demonstrate that the protective effect of low dose (20 µg) of ODN 1826 drug preconditioning is not due to a lower drop in CBF during ischemia.
In a new window | Download PPT
Figure 3: (A) Infarct volume and (B) drop in CBF during MCAO in 8 mice belonging to the drug-treated group where we observed a maximum drop in CBF and 8 mice belonging to the drug-treated group where we observed a minimum drop in CBF. Data presented in this figure also include data presented from male mice in Figures 2A-B. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
Low dose of CpG-ODN1826 protects young male but not young Ovx female mice against cerebral ischemia
We performed the next set of experiments to address an additional STAIR recommendation of testing the efficacy of the CpG-ODN1826 preconditioning agent in female mice. Our study included Ovx female mice to avoid the potential impact of estrous cycle-related hormonal changes on cerebral ischemic outcomes. For these experiments we included four groups of animals: 1) young male mice treated with 20 µg ODN 1826 control, 2) young male mice treated with 20 µg ODN 1826 drug, 3) young Ovx female mice treated with 20 µg ODN 1826 control, and 4) young Ovx female mice treated with 20 µg ODN 1826 drug. Similar to the first experiment described above, mice were treated with ODN 1826 control or ODN 1826 drug 3 days prior to the induction of MCAO. Ovx female mice were purchased from Jackson Laboratories and were 8 - 12 weeks old at the time of experiment. The duration between Ovx surgery and MCAO surgery was at least one week.
CBF during MCAO for male mice treated with 20 µg ODN 1826 control, male mice treated with 20 µg ODN 1826 drug, Ovx female mice treated with 20 µg ODN 1826 control, and Ovx female mice treated with 20 µg ODN 1826 drug dropped to 9.3 ± 0.9, 13.1 ± 0.9, 11.4 ± 1.1, and 10.9 ± 1.2% of baseline CBF, respectively (Figure 4A). The infarct volume in male mice treated with 20 µg ODN 1826 drug was significantly lower (31 ± 3%, n = 13) compared to male mice treated with 20 µg ODN 1826 control (39 ± 2%, n = 14) (Figure 4B). The infarct volumes in Ovx female mice treated with 20 µg ODN 1826 control and Ovx female mice treated with 20 µg ODN 1826 drug were 37 ± 2% (n = 18) and 37 ± 3% (n = 16), respectively (Figure 4B). There were no significant differences between the two Ovx female groups.
In a new window | Download PPT
Figure 4: A comparative study evaluating the effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia. Groups included: 1) young male mice treated with 20 µg ODN 1826 control (n = 14), 2) young male mice treated with 20 µg ODN 1826 drug (n = 13), 3) young Ovx female mice treated with 20 µg ODN 1826 control (n = 18), and 4) young Ovx female mice treated with 20 µg ODN 1826 drug (n = 16). Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
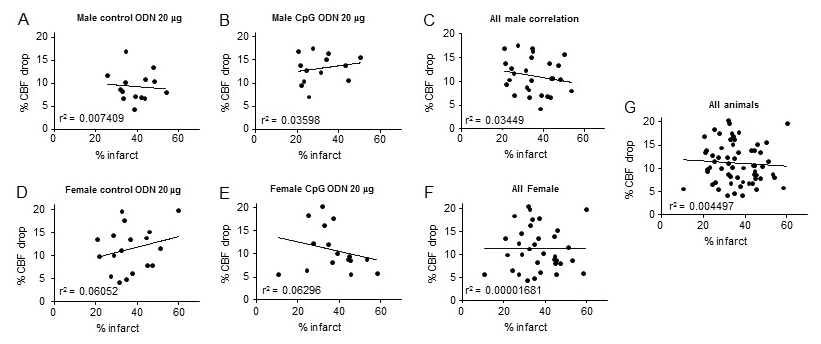
Because the drop in CBF during MCAO was significantly different between male mice treated with 20 µg ODN 1826 control and male mice treated with 20 µg ODN 1826 drug, we determined a correlation between the drop in CBF and infarct volume. These results are presented in Figure 5A-G. Overall, since the drop in CBF during ischemia was in a very narrow range, we did not see any significant correlation between the drop in CBF and infarct volume. These results suggest that the observed protective effect of low dose ODN 1826 drug in male mice is not due to a lower drop in CBF during ischemia.
In a new window | Download PPT
Figure 5: Graphs showing correlation between infarct volume and drop in CBF during MCAO in different experimental groups. Graph A – G contains results of animals belonging to (A) young male mice treated with 20 µg ODN 1826 control (n = 14), (B) young male mice treated with 20 µg ODN 1826 drug (n = 13), (C) all young male mice (n = 27), (D) young Ovx female mice treated with 20 µg ODN 1826 control (n = 18), (E) young Ovx female mice treated with 20 µg ODN 1826 drug (n = 16), (F) all young Ovx female mice (n = 34), and (G) all male and female animals (n = 61). R2 values are provided on respective graphs.
Discussion
Induction of ischemic tolerance in a clinical setting by exposing patients with a high risk for recurrent ischemic attacks to sub-lethal cerebral ischemia is impractical. However, pharmacological conditioning to induce ischemic tolerance in a clinical setting is feasible. As mentioned in the introduction, there are multiple pharmacological strategies that demonstrated promising results in the induction of ischemic tolerance in preclinical studies. Considering several neuroprotective strategies failed in clinical settings, the emphasis has been made on independent replication of preclinical studies before moving onto expensive clinical testing (Stroke Therapy Academic Industry, 1999; Bosetti et al., 2017). The goal of our study was to independently replicate an earlier finding of CpG-ODN 1826 preconditioning-induced neuroprotection (Stevens et al., 2008). Our results demonstrate that we could successfully replicate the above-mentioned study by demonstrating that a low dose (20 µg/mouse) of CpG ODN 1826 is able to induce tolerance in male mice against transient focal cerebral ischemia-induced damage. This type of replication approach was fostered by NINDS as a mechanism of potentially moving forward CpG ODN 1826 into the clinic as proposed by the Stenzel-Poore lab. This approach has similarities with the new strategy of the Stroke Preclinical Assessment Network (SPAN) program launched by NINDS in 2019, where multiple laboratories will replicate findings of a potential neurotherapeutic drug before potentially taking this treatment to clinical trials.
The present study used young mice to evaluate the ischemic tolerance-inducing efficiency of CpG-ODN 1826. Since aging is one of the major risk factors for stroke, future studies should include aged animals of both sexes. Comorbidities such as diabetes, hypertension, obesity, and hypercholesterolemia are known risk factors for stroke. Since ischemic tolerance pathways may overlap with pathways activated by these comorbidities, overactivation of those pathways with preconditioning stimuli may result in no benefit or worsening of post-stroke outcomes in these comorbid conditions. For example, earlier review articles highlighted that diabetes reduces the efficiency of pharmacological preconditioning in inducing ischemic tolerance and studies using models of diabetes demonstrated the loss of conditioning-induced cardioprotection (Rehni and Dave, 2018; Wider and Przyklenk, 2019). Considering this, evaluating the efficacy of CpG-ODN 1826 in animal models of these comorbidities should also be considered prior to its testing in a clinical settings. The present study evaluated short-term outcomes following induction of cerebral ischemia. It is possible that protective effects may lessen or disappear with long-term post-stroke survival. The efficacy of CpG-ODN 1826 on post-stroke long-term outcomes (both histopathological and functional) also remains to be evaluated.
In the second half of our studies, we tested the efficacy of CpG-ODN1826 preconditioning agent in female mice. We used young Ovx female mice to test the efficacy of CpG-ODN 1826 to avoid the potential impact of estrous cycle-related hormonal changes on cerebral ischemic outcomes. The infarct volume in Ovx female mice treated with ODN 1826 control and with ODN 1826 drug showed no significant differences between the two groups. Our results demonstrate that CpG-ODN1826 preconditioning is efficacious in inducing tolerance against cerebral ischemia in male but not in Ovx female mice.
There are some potential explanations for the lack of protection. In females, there exists a complex relationship between the ovaries, the immune system, and the central nervous system that configures the immune response within the brain. The ovarian hormones estrogen and progesterone exert potent immunomodulatory effects on the whole body, including the brain (Mor et al., 1999; Vegeto et al., 2003; Brown et al., 2010; Sarvari et al., 2011). Estradiol-17β (E2; a potent estrogen) suppresses the expression of genes associated with the innate immune system in the cortex of Ovx rodents (Sarvari et al., 2010). E2 exerts its effects on the brain (both neurons and glia) via activation of estrogen receptor subtype alpha or beta (ERs), and protein levels of these receptors decline following either Ovx or reproductive senescence in the brain of female rodents (Nilsson et al., 2011; Waters et al., 2011). In a previously published study, we demonstrated that ER beta regulates activation of a key innate immune component inflammasome in the brain of female rats (de Rivero Vaccari et al., 2016). The levels of the inflammasome proteins caspase 1, apoptosis-associated speck-like protein containing a CARD (ASC), and interleukin (IL)-1β were lower in the brain of young compared to middle-aged reproductively senescent female rats. Aforementioned studies suggested a role of ERs on neuroinflammation; however, it did not identify whether ERs mediated regulation of inflammation occurs at the level of neurons, glia, or both (Raval et al., 2019). Additional confirmation to the notion that ERs regulate innate response in the brain comes from studies showing that ERs are present near the genes encoding selected TLR (Rettew et al., 2009; Liu et al., 2014). Microglial deletion of the ER-alpha DNA binding site blocks pathogen-associated molecular pattern (PAMPs)/damage-associated molecular pattern (DAMPs) up-regulation of TLR, which suggests that estrogens increase the ability of microglia to respond to noxious stimuli (Vegeto et al., 2008; Cunningham et al., 2014b; Cunningham et al., 2014a; Villa et al., 2016). It is also known that estrogens inhibit the production of inflammatory cytokines by interfering with TLR signaling through NF-κB (Villa et al., 2016). Therefore, future studies will be needed to understand the interaction between ERs and innate immune component/TLRs. Furthermore, our approach using Ovx-ovarian hormonal deprived-mice with the intent to avoid influence of cyclic ovarian hormones on the brain might have prevented the modulatory effects of E2/ERs on the TLR ligand CpG-ODN 1826, blocking CpG-ODN 1826-induced ischemic protection. Future studies using young ovary-intact female mice taking into account the estrous cycle stages will be required. This is also important because the normal estrous cycle induced E2 fluctuations affects post-ischemic neuronal survival in young rodents (Raval et al., 2009; de Rivero Vaccari et al., 2019). In addition, women’s risk of stroke increases after reaching menopause, thus testing the efficacy of CpG-ODN 1826 preconditioning in reproductively senescent and aged mice will be important. Such information will also be in tune with the STAIR consortium recommendation that “efficacy studies should be performed in both male and female animals” and clinically relevant for future translation of CpG-ODN 1826.
As mentioned above, we did not observe a protective effect of CpG-ODN 1826 preconditioning on cerebral ischemic damage in Ovx female animals. The potential sex difference in the efficacy of various preconditioning stimuli in inducing ischemic tolerance in various organ systems have been evaluated by earlier studies. For example, an earlier study evaluating the impact of ischemic preconditioning on muscle performance, and hemodynamic and O2 uptake during voluntary knee extensions in strength-trained male and female participants observed that males benefited more than females (Paradis-Deschenes et al., 2016). Similarly, a study by Ledvenyiova et al., (2013) using an in vitro model of myocardial ischemia/reperfusion, demonstrated that ischemic preconditioning was effective in reducing the size of myocardial infarction in the heart belonging to both male and female rats (Ledvenyiova et al., 2013). On the other hand, another group using a similar in vitro model of myocardial ischemia/reperfusion reported that ischemic preconditioning was able to reduce the infarct size in the hearts of male but not female mice (Song et al., 2003). Isoflurane preconditioning decreased cerebral ischemic damage in young and middle-aged male mice but increased cerebral ischemic damage in young female mice and had no impact in middle-aged female mice (Kitano et al., 2007). The same group of investigators also evaluated the impact of in vitro isoflurane preconditioning on oxygen and glucose deprivation (OGD)-induced neuronal death. They observed that isoflurane preconditioning was able to increase cell survival following OGD regardless of innate cell sex (Johnsen and Murphy, 2011). Hoda and colleagues (2012; 2014) demonstrated that remote ischemic perconditioning protects the brain against cerebral ischemic damage in Ovx female animals similar to their earlier study in male animals. It appears differences in the type of preconditioning stimuli, target organ system, and levels of sex hormones at the time of induction of conditioning may explain the observed sex differences in the above studies. However, further detailed studies are required to define the sex-specific effect of conditioning on cerebral ischemic damage.
In conclusion, our results independently confirm the protective effect of CpG-ODN 1826 in inducing cerebral ischemic tolerance. The protective effect of CpG-ODN 1826 appears dose and sex specific, as we did not observe any protection at a higher dose in young male animals or in Ovx female animals. It is plausible that a different dose of CpG-ODN 1826 may be required to induce ischemic tolerance in young Ovx female mice. Considering other STAIR recommendations, future studies confirming the efficacy of CpG-ODN 1826 in aged animals and animals with comorbidities commonly observed in stroke patients may help further support testing its efficacy in a clinical setting.
Acknowledgment:
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grant NS34773 (to M.A.P.P.).
References
Kunjan R. Dave1,2,3
1Peritz Scheinberg Cerebral Vascular Disease Research Laboratories, 2Department of Neurology and 3Neuroscience Program, University of Miami School of Medicine, Miami, FL, USA.
Isabel Saul1,2
1Peritz Scheinberg Cerebral Vascular Disease Research Laboratories, 2Department of Neurology, University of Miami School of Medicine, Miami, FL, USA.
Ami P. Raval1,2
1Peritz Scheinberg Cerebral Vascular Disease Research Laboratories, 2Department of Neurology, University of Miami School of Medicine, Miami, FL, USA.
Miguel A. Perez-Pinzon1,2,3
1Peritz Scheinberg Cerebral Vascular Disease Research Laboratories, 2Department of Neurology and 3Neuroscience Program, University of Miami School of Medicine, Miami, FL, USA.
Corresponding author:
Miguel A. Perez-Pinzon
Email: perezpinzon@med.miami.edu
In a new window | Download PPT
Figure 1: The effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia. Groups included 1) saline (n = 11), 20 µg ODN 1826 control (n = 13), 20 µg ODN 1826 (n = 15), 40 µg ODN 1826 control (n = 9), and 40 µg ODN 1826-treated (n = 10) young male mice. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
In a new window | Download PPT
Figure 2: The protective effect of CpG-ODN1826 preconditioning in a larger number of young male mice (n = 20/group). The effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia with an increased sample size. Groups included 1) 20 µg ODN 1826 control (n = 20) and 20 µg ODN 1826-treated (n = 20) young male mice. Data presented in this figure also include data presented from male mice in Figures 1A-B. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
In a new window | Download PPT
Figure 3: (A) Infarct volume and (B) drop in CBF during MCAO in 8 mice belonging to the drug-treated group where we observed a maximum drop in CBF and 8 mice belonging to the drug-treated group where we observed a minimum drop in CBF. Data presented in this figure also include data presented from male mice in Figures 2A-B. Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
In a new window | Download PPT
Figure 4: A comparative study evaluating the effect of CpG ODN 1826 preconditioning on (A) CBF drop during ischemia and (B) infarct size in a mouse model of focal ischemia. Groups included: 1) young male mice treated with 20 µg ODN 1826 control (n = 14), 2) young male mice treated with 20 µg ODN 1826 drug (n = 13), 3) young Ovx female mice treated with 20 µg ODN 1826 control (n = 18), and 4) young Ovx female mice treated with 20 µg ODN 1826 drug (n = 16). Results are presented as mean ± SEM. Observed significant differences are indicated by p values.
In a new window | Download PPT
Figure 5: Graphs showing correlation between infarct volume and drop in CBF during MCAO in different experimental groups. Graph A – G contains results of animals belonging to (A) young male mice treated with 20 µg ODN 1826 control (n = 14), (B) young male mice treated with 20 µg ODN 1826 drug (n = 13), (C) all young male mice (n = 27), (D) young Ovx female mice treated with 20 µg ODN 1826 control (n = 18), (E) young Ovx female mice treated with 20 µg ODN 1826 drug (n = 16), (F) all young Ovx female mice (n = 34), and (G) all male and female animals (n = 61). R2 values are provided on respective graphs.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9610 | 3 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA