International bi-monthly journal of cell signaling, tissue protection, and translational research.
Clinical practice guidelines of remote ischemic conditioning for the management of cerebrovascular diseases
Xunming Ji1, Wenbo Zhao2,3, Johannes Boltze4, Sijie Li3, Ran Meng2, Yuan Wang2, Gregory J Bix5, Cesar V Borlongan6, Jeffrey M Gidday7, Sebastian Koch8, John C Quindry9, Rajiv R Ratan10, Kristin Veighey11,12, Guohua Xi13, Giuseppe Pignataro14, David C Hess15, Derek J Hausenloy16,17,18,19,20,21
Author Affiliations


- 1Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 3Beijing Key Laboratory of Hypoxic Conditioning Translational Medicine, Xuanwu Hospital, Capital Medical University, Beijing, China
- 4School of Life Sciences, University of Warwick, Coventry CV4 7AL, United Kingdom
- 5Clinical Neuroscience Research Center, Tulane University School of Medicine, New Orleans, LA USA
- 6Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, FL USA
- 7Departments of Ophthalmology, Physiology, and Neuroscience Louisiana State University School of Medicine, New Orleans, LA
- 8Department of Neurology, University of Miami Miller School of Medicine, Miami, FL, USA
- 9School of Integrative Physiology and Athletic Training, College of Health Professions and Biomedical Sciences University of Montana, Missoula, MT
- 10Burke Medical Research Institute, White Plains, NY USA
- 11Wessex Kidney Centre, Queen Alexandra Hospital, Portsmouth, UK
- 12Research & Development, University Hospital Southampton NHS Foundation Trust, Southampton, UK
- 13Department of Neurosurgery, University of Michigan, Ann Arbor, MI USA
- 14Department of Neuroscience, School of Medicine “Federico II” University of Naples, Napoli, Italy
- 15Medical College of Georgia, Augusta University, Augusta, GA USA
- 16Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore
- 17National Heart Research Institute Singapore, National Heart Centre, Singapore
- 18Yong Loo Lin School of Medicine, National University Singapore, Singapore
- 19The Hatter Cardiovascular Institute, University College London, London, UK
- 20The National Institute of Health Research University College London Hospitals Biomedical Research Centre, Research & Development, London, UK
- 21Department of Cardiology, Barts Heart Centre, St Bartholomew’s Hospital, London, UK
Abstract
Remote ischemic conditioning (RIC) using transient limb ischemia and reperfusion has been shown in small clinical studies to reduce myocardial injury and infarction in cardiac patients, although larger clinical outcome studies have been neutral. Experimental and emerging clinical studies have also reported beneficial effects of limb RIC in a number of different settings of cerebrovascular disease including stroke (ischemic and hemorrhagic), carotid artery stenosis, intracranial artery stenosis, aneurysms, small vessel disease, and vascular cognitive impairment. Although limb RIC has many advantages, in that it is non-invasive, easy to administer, relatively innocuous, cost-effective, has few or no contraindications, and may be deployed under various circumstances (e.g., home, ambulance, and hospital), several questions remain regarding its clinical application for cerebrovascular disease. Therefore, in this document, we aim to provide practicing clinicians with a coherent synthesis of the latest scientific evidence, and we propose several recommendations to help facilitate the clinical application of limb RIC for the management of cerebrovascular disease.
Keywords: clinical practice guidelines, remote ischemic conditioning, preconditioning, cerebrovascular diseases, stroke, cognitive impairment.
Abstract
Remote ischemic conditioning (RIC) using transient limb ischemia and reperfusion has been shown in small clinical studies to reduce myocardial injury and infarction in cardiac patients, although larger clinical outcome studies have been neutral. Experimental and emerging clinical studies have also reported beneficial effects of limb RIC in a number of different settings of cerebrovascular disease including stroke (ischemic and hemorrhagic), carotid artery stenosis, intracranial artery stenosis, aneurysms, small vessel disease, and vascular cognitive impairment. Although limb RIC has many advantages, in that it is non-invasive, easy to administer, relatively innocuous, cost-effective, has few or no contraindications, and may be deployed under various circumstances (e.g., home, ambulance, and hospital), several questions remain regarding its clinical application for cerebrovascular disease. Therefore, in this document, we aim to provide practicing clinicians with a coherent synthesis of the latest scientific evidence, and we propose several recommendations to help facilitate the clinical application of limb RIC for the management of cerebrovascular disease.
Keywords: clinical practice guidelines, remote ischemic conditioning, preconditioning, cerebrovascular diseases, stroke, cognitive impairment.
Introduction
Cerebrovascular disease refers to a wide spectrum of disorders of the brain vasculature, including stroke (ischemic and hemorrhagic), carotid artery stenosis, intracranial artery stenosis, aneurysms, small vessel disease, and vascular cognitive impairment (Fisher, 2010; Rea, 2015; Smith et al., 2017; Absher et al., 2018). Currently, cerebrovascular disease is the second most common cause of mortality worldwide and the most common cause of long-term disability (Lozano et al., 2012; Vos et al., 2017). In some regions of the world, it has exceeded ischemic heart disease as the leading cause of death (Roth et al., 2015a). Consequently, cerebrovascular disease is responsible for great disease burden worldwide (Hay et al., 2017; Vos et al., 2017; Wang et al., 2017a). In the past four decades, the diagnosis and treatment of cerebrovascular disease has advanced significantly. Furthermore, studies have revealed that approximately 90% of cerebrovascular disease cases are preventable with medications, surgery, and modifications of risk factors (O'Donnell et al., 2010; Caprio and Sorond, 2019). Despite improvements in preventive and therapeutic measures, their effects on cerebrovascular disease remain far below expectation. Furthermore, many of the treatments incur significant costs and are associated with serious complications (Rose, 1981; Feigin et al., 2015b; Benjamin et al., 2019). Thus, cerebrovascular disease burden continues to increase worldwide (Feigin et al., 2015a; Roth et al., 2015b). These collective issues have created an urgent, unmet need for new effective and safe strategies for the management of cerebrovascular disease.
Remote ischemic conditioning
Remote ischemic conditioning (RIC) has evolved from classic ischemic preconditioning (Murry et al., 1986), and is a protective systemic strategy whereby several cycles of brief non-lethal ischemia followed by reperfusion in an organ or tissue confer protection against subsequent, more severe lethal ischemia in distant vital organs (e.g., heart and brain) (Przyklenk et al., 1993). After decades of methodological evolution, RIC is now generally simply performed on the arms or legs using blood pressure cuffs inflated to induce transient ischemia and reperfusion (Hess et al., 2015; Hausenloy et al., 2016; Hausenloy and Yellon, 2016). Based on investigations of coronary heart disease, RIC has now been widely investigated in many other organs, including brain, kidney, lung, liver, and the gastrointestinal tract (Holscher et al., 2007; Crowley and McIntyre, 2013; Li et al., 2014; Chu et al., 2015; Hausenloy and Yellon, 2016; Garcia-de-la-Asuncion et al., 2017; Ghelfi et al., 2017; Zhao et al., 2019). As cerebrovascular disease and coronary heart disease share many common pathophysiological mechanisms, an increasing number of RIC studies have focused on cerebrovascular disease management. To date, RIC has been shown to increase cerebral tolerance to ischemic injury, reduce the risk of cerebral infarction, improve cerebral perfusion status, and promote the formation of cerebral collaterals in patients with ischemic stroke (Meng et al., 2012; Hougaard et al., 2014; Meng et al., 2017b). Furthermore, RIC also can be safely used in patients with intracranial hemorrhage and can improve their functional outcomes (Koch et al., 2011; Gonzalez et al., 2014; Laiwalla et al., 2016). As a non-invasive, safe strategy, RIC has paved the way for exciting new prospects in the broader management of both ischemic and hemorrhagic cerebrovascular disease (Hess et al., 2015; Zhao et al., 2019).
Why it is important to develop these clinical practice guidelines
Based on current studies, RIC is non-invasive, easy to administer, innocuous, cost-effective, has few or no contraindications, and may be deployed under various circumstances (e.g., home, ambulance, and hospital) (Zhao et al., 2019). However, many questions regarding the use of RIC for cerebrovascular disease management have been left unanswered, such as: 1) “How many types of RICs exist?; 2) How might RIC work?; 3) Which subtypes of cerebrovascular disease patients should RIC be implemented, and how?; 4) What should be done prior to the initiation of RIC treatment to ensure its safety?; 5) Are there any contraindications or adverse events related to RIC?; and 6) Which device should be used to perform RIC procedures? These collective questions might hinder the broad acceptance of RIC by clinicians, investigators, and patients as an effective strategy for the management of cerebrovascular disease.
This document aims to provide practicing clinicians with a coherent synthesis of the latest research and several recommendations to facilitate the clinical application of RIC for the management of cerebrovascular disease. Because the clinical questions surrounding the management of cerebrovascular disease with RIC can be broad and beyond the scope of a single document, this panel of experts opted to narrow the focus of the guidelines to critical issues of the utmost relevance to clinicians, patients, and caregivers.
Methods
Committee composition
The guideline development panel encompassed multidisciplinary specialists, including neuroscientists, stroke neurologists, cognitive neurologists, neurosurgeons, neuroradiologists, and cardiologists. Three patients who received RIC treatment and their primary caregivers also provided insightful suggestions used to formulate the clinical questions. Beside suggestions from the panel of practicing clinicians, investigators, patients, and caregivers, the panel also employed the GRADE approach (Grading of Recommendations, Assessment, Development, and Evaluation) to formulate clinical questions II through VI, to summarize the relevant evidence, and to develop recommendations for clinical practice. Full methodological details and tables supporting the recommendations for these five clinical questions can be found in the online supplement. To address clinical question I, the panel of experts identified both animal and clinical studies, and subsequently synthesized the latest evidence. In addition, instead of a systematic literature review, the expert panel performed a narrative review of the evidence to identify the best answers to clinical questions VII and XI. For clinical questions VIII, IX, and X, recommendations were based on a consensus of expert opinion, as there were no specific studies investigating those questions.
Clinical question I: How many subtypes of RIC are there and how might they work?
Subtypes of RIC
The first RIC report was published by Przyklenk, Whittaker, and colleagues in 1993. They reported the novel finding that several brief periods of ischemia followed by reperfusion applied to the myocardium supplied by one coronary branch conferred resistance against lethal ischemia/reperfusion to another part of the myocardium supplied by a different coronary branch and reduced the final infarct size (Przyklenk et al., 1993). This intriguing phenomenon was named “Remote Ischemic Preconditioning”. In 2003, Dr. Zhiqing Zhao from Vinten-Johansen’s group first reported “Ischemic Postconditioning,” based on the attenuation of reperfusion injury with a conditioning procedure commenced only at the start of reperfusion in a canine model of myocardial infarction (Zhao et al., 2003). Next, in 2005, Vinten-Johansen’s group reported that brief renal ischemia and reperfusion applied before coronary artery reperfusion reduced myocardial infarct size by activation of adenosine receptors, and this endogenous protection was called “Remote Postconditioning” (Kerendi et al., 2005). However, the “Remote Postconditioning” reported in this particular study is now defined as “Remote Ischemic Perconditioning”, as the conditioning stimulus was applied during the index myocardial ischemic episode. Li and colleagues reported in 2006 that limb ischemic postconditioning protects the myocardium from ischemia-reperfusion injury (Li et al., 2006), providing the most promising type of postconditioning for clinical application to date. In 2007, Andreka et al., (2007) reported that remote ischemic postconditioning induced by four 5 minutes cycles of blood pressure cuff inflation applied to the lower limb immediately after reperfusion significantly reduced myocardial infarct size; this was the first time that the term “Remote Ischemic Postconditioning” was formally used. In the same year, Schmidt and colleagues conducted an animal study to test the protective effects of ischemic preconditioning when it was administered during ischemia and before reperfusion (i.e., perconditioning) (Schmidt et al., 2007). Based on this study, the existence of remote ischemic perconditioning was first shown and demonstrated to be effective.
Currently, the term “remote ischemic conditioning” is often employed to encompass remote ischemic pre-, per-, and postconditioning. These three subtypes of RIC were proposed based on models of myocardial ischemia/reperfusion. Remote ischemic preconditioning is applied before the index ischemia, remote ischemic perconditioning is applied after ischemia but before reperfusion of the index ischemia, and remote ischemic postconditioning is applied after reperfusion of the index ischemia (Hess et al., 2015). In clinical practice, the terms can be used accurately in acute ischemic stroke patients treated with endovascular thrombectomy and in acute myocardial infarction patients treated with percutaneous coronary intervention. In other patient populations, however, it may be difficult to clearly distinguish between subtypes of RIC. Therefore, the term Remote Ischemic Conditioning, while not necessarily precise, appears to be the best compromise.
Mechanisms underlying RIC
RIC has been widely investigated in both animal and human studies, but the precise mechanisms through which it exerts protection against ischemic insults in a distant organ or tissue is currently unclear. The mechanisms underlying RIC have been studied for nearly 30 years, and most of the published literature on RIC is related to the heart. Based on this large body of work, the potential mechanisms underlying RIC can be classified into four categories—humoral, neural, immune, and inflammatory pathways.
It is known that a period of reperfusion of the remote conditioned organ or tissue is essential for RIC, suggesting transportation of humoral/diffusible factors produced during the ischemic period to distant organs (Gho et al., 1996). In addition, the use of a ganglion blocker (hexamethonium) (Gho et al., 1996), resection of the neural innervation of the limb (Lim et al., 2010), and genetic inhibition of preganglionic vagal neurons in the brainstem (Mastitskaya et al., 2012), have all been shown to abrogate the protection of RIC. These findings indicate that an intact neural pathway is also required for RIC. Furthermore, stimulation of neural pathways in the remotely conditioned organ and tissue may be elicited by local production of autacoids, such as adenosine (Steensrud et al., 2010), bradykinin (Schoemaker and van Heijningen, 2000), calcitonin gene-related peptide (Li et al., 1996), and others. Although neurohumoral factors may initiate the cascade of conditioning and produce a preconditioning-like effect, it also appears that non-specific innate immunity may be instrumental in ischemic conditioning. The key elements of the latter process are gene expression, leukocytes, and mitochondria. Several animal studies have examined the effects of RIC on inflammatory responses and observed that the pro-inflammatory response is inhibited while a favorable profile of anti-inflammatory and anti-apoptotic gene transcription is engaged (Tsang et al., 2005; Fujita et al., 2007; Shi and Vinten-Johansen, 2012).
Importantly, studies have found that RIC improves collaterals and cerebral blood flow, which may play important roles in reducing cerebral damage. Experimental studies have found that RIC promoted new collateral formation and angiogenesis and augmented collateral flow, and this has been demonstrated to be why RIC reduces cerebral damage and improve neurological outcomes (Ma et al., 2017; Kitagawa et al., 2018; Zhang et al., 2019). A clinical study also found that in patients with symptomatic intracranial atherosclerotic stenosis, long-term RIC appears to accelerate cerebral collateral opening and vascular remodeling, which may play roles in preventing recurrent ischemic stroke (Meng et al., 2017a). Additionally, RIC has been found to improve cerebral blood flow in both ischemic and hemorrhagic stroke models (Laiwalla et al., 2016; Ren et al., 2018), and this phenomenon has been further demonstrated in patients with ischemic cerebrovascular diseases (Meng et al., 2012).
In summary, the mechanisms that mediate RIC are multifactorial, and the precise interrelationship between various signals is not yet clearly defined. Although the potential mechanisms mediating RIC are often divided into three separate categories, it is important to note that these three pathways may interact or overlap, and are not mutually exclusive.
Clinical question II: Should RIC be used to improve functional outcomes in patients with acute ischemic stroke?
Background. Acute ischemic stroke is caused by occlusion of a cerebral artery; therefore, timely recanalization of the occluded artery and reperfusion of salvageable ischemic brain tissue are the most effective maneuvers for salvaging tissue that is not already infarcted (Rha and Saver, 2007; Prabhakaran et al., 2015). Currently, intravenous thrombolysis and endovascular thrombectomy are standard strategies for recanalization therapy. However, only a small proportion of acute ischemic stroke patients with large-vessel occlusion actually receive thrombectomy (approximately 10%, even in the USA) (McMeekin et al., 2017; Rai et al., 2017; Rocha and Jovin, 2017). Even worse, with these therapies, therapeutic effects are far from satisfactory (Campbell et al., 2015; Fisher and Saver, 2015; Goyal et al., 2015; Jovin et al., 2015; Saver et al., 2015; Goyal et al., 2016; Zhao et al., 2017a; Zhao et al., 2018d). Thus, there still remains ample room for improvement in patients with acute ischemic stroke, and alternative or adjunctive therapeutic strategies are urgently needed.
Summary of Evidence. The literature search revealed four published studies, one recently completed study, and one systematic review pertinent to this clinical question (Table 1). One study assessed RIC in acute ischemic stroke patients treated with intravenous thrombolysis, one study assessed RIC in acute stroke patients treated with reperfusion therapy, another assessed the safety and feasibility of RIC in patients treated with thrombectomy, and two other studies assessed RIC in patients with acute ischemic stroke who did not receive any reperfusion therapies.
Table 1. Remote ischemic conditioning (RIC) in patients with cerebrovascular disease.
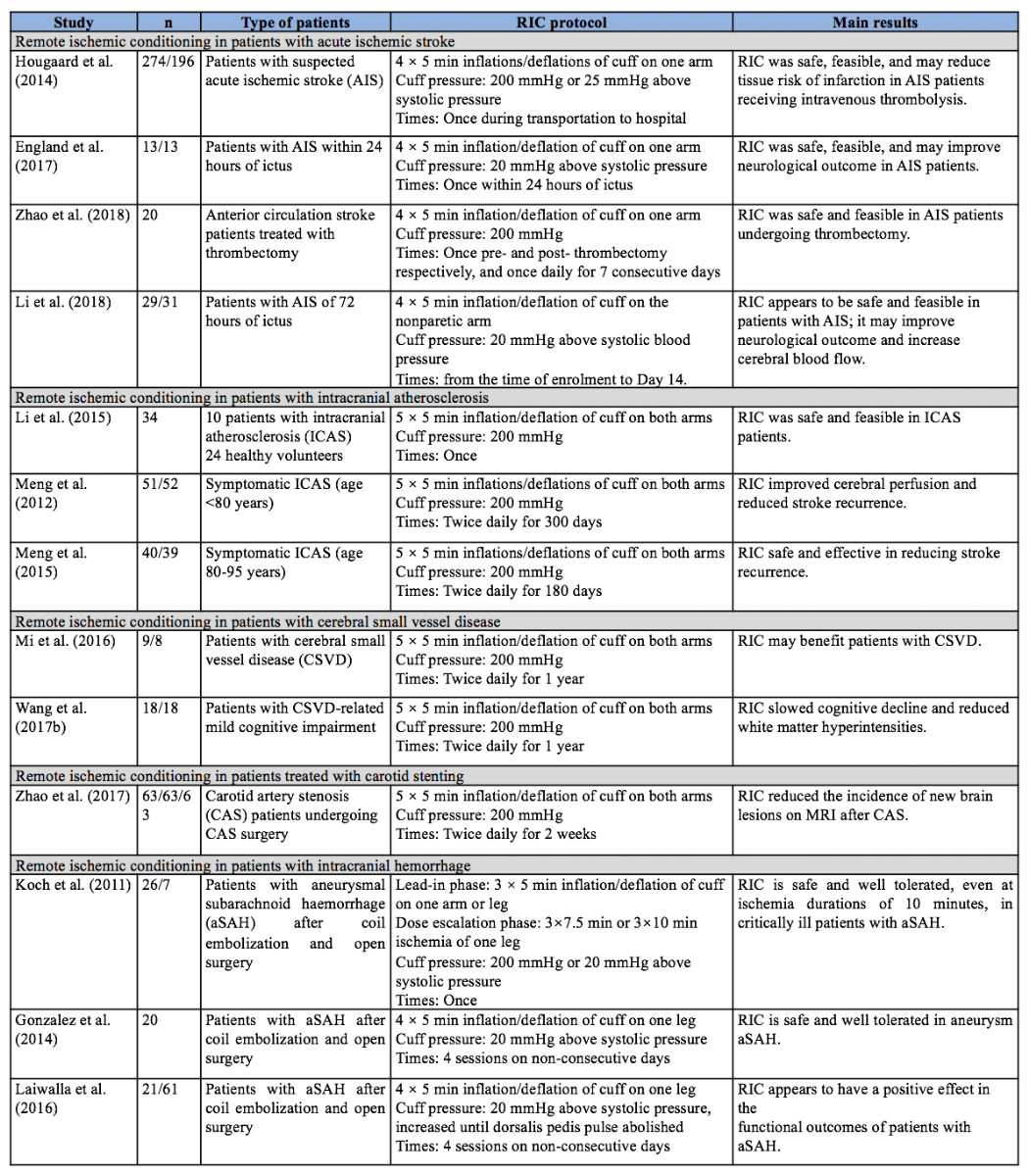
A Phase I study named REVISE-1 assessed the safety and feasibility of RIC in patients with anterior circulation stroke who were treated with endovascular thrombectomy within six hours of ictus (Zhao et al., 2018c). Twenty patients were recruited and underwent RIC pre- and post-thrombectomy, and then once daily for 7 consecutive days. RIC was well tolerated and feasible in this patient population; it exerted no significant influence on vital signs (i.e., blood pressure and heart rate), intracranial pressure, cranial prefusion pressure, or the peak velocity of the middle cerebral artery.
A proof-of-concept randomized controlled trial (RCT) investigated the impact of prehospital RIC as adjunctive therapy for acute ischemic stroke patients (≥ 18 years old) who were candidates to receive intravenous thrombolysis within 4.5 hours of symptom onset (Hougaard et al., 2013; Hougaard et al., 2014). Four cycles of RIC stimulation were performed by the ambulance staff during transportation and, if not completed, the procedure was discontinued upon arrival at the stroke unit. Among the patients with confirmed acute ischemic stroke who received intravenous thrombolysis, there was no difference between the RIC (n = 91) and the control (n = 80) groups, with respect to penumbral salvage, final infarct size, infarct growth over baseline, or clinical outcomes at 3 months. However, in patients treated with RIC, a large proportion of the hypoperfusion area clearly displayed a lower risk of infarction at 1 month when compared with the controls (likelihood ratio test P = 0.0003).
Another RCT named RECAST-1 assessed the safety and primary efficacy of RIC in patients with acute ischemic stroke within 24 hours of ictus (England et al., 2017). Twenty-six patients with acute ischemic stroke were recruited and allocated to receive four cycles of RIC stimulus or sham RIC stimulus in the nonparetic arm. RIC was safe and feasible in this patient population, and 90-day National Institutes of Health Stroke Scale (NIHSS) scores were significantly lower in those receiving RIC (median 1 [interquartile range, 0.5-5] versus median 3 [interquartile range, 2-9.5], P = 0.04).
One study assessed RIC in patients with acute ischemic stroke within 72 hours of ictus. Sixty patients were recruited and divided into two groups receiving either RIC or sham RIC up to day 14 (Li et al., 2018). At the 3-month follow-up appointment, RIC elicited a significant decrease in the NIHSS score and a 31.3% decrease in infarct volumes compared with the sham RIC control group (p < 0.05).
The recently completed REmote iSchemic Conditioning in acUtE BRAin INfarction Study (RESCUE-BRAIN) failed to demonstrate any benefit with lower limb RIC in terms of reducing cerebral infarct size at 24 hours (on MRI diffusion weighted imaging) in patients presenting with an acute ischemic stroke and treated by thrombolysis (ClinicalTrials.gov Identifier: NCT02189928). No benefits were observed with RIC on secondary functional endpoints including NIHSS scores at 24 hours, and modified Rankin Scale (mRS) scores at 90 days.
In the meta-analysis of RIC for ischemic stroke (Zhao et al., 2018b), studies of patients with acute ischemic stroke were also included. However, the results showed that, for patients with acute ischemic stroke, there was no significant difference between RIC and non-RIC groups in stroke severity, as assessed by the NIHSS score and the final infarct volume (standardised mean difference (SMD) -0.24 mL, 95% CI -1.02 to 0.54).
Panel Recommendations.
In patients with acute ischemic stroke, RIC can be considered in those not receiving reperfusion therapy, however, currently available evidence suggests that RIC should not be used as routine adjunctive therapy in those receiving reperfusion therapy (conditional recommendation, low confidence in estimate of effects; see E-Table A1 and E-Table B1 in the Online Supplement).
Discussion.
Justification. Among these five clinical studies, two studies showed RIC could improve functional outcome 90 days later in acute ischemic stroke patients that did not receive reperfusion therapy, therefore, RIC can be considered to improve neurological outcomes in those patients. Three clinical trials employed RIC in acute stroke patients that were treated with reperfusion therapy, but all three studies failed to prove the safety and efficacy profile of RIC under these conditions. Application of RIC as adjunctive therapy in acute ischemic stroke patient receiving reperfusion appears to be too early.
Future studies. Although remote ischemic perconditioning during transportation to the hospital may preserve salvageable tissue for reperfusion therapy (Hougaard et al., 2014), if the occluded arteries are not eventually recanalized, the salvaged cerebral tissue will likely succumb to infarct. Therefore, acute ischemic stroke patients receiving endovascular therapy might be optimal candidates for investigations of the neuroprotective effects of RIC, as they can achieve a higher rate of recanalization (60-90%). Zhao and colleagues (2018c) have demonstrated the safety and feasibility of RIC in patients treated with thrombectomy, but this study recruited only 20 patents and did not examine the occurrence of hemorrhagic transformation. In addition, two studies showed RIC could improve functional outcomes in patients not receiving reperfusion therapy, however these two studies only recruited a small sample size and the power was low. Therefore, larger efficacy trials are warranted to determine both the safety profiles and efficacy against acute ischemic stroke in patients with or without reperfusion therapy. Furthermore, the benefits of RIC against cerebral infarct volume should be confirmed.
Clinical question III: Should RIC be used to reduce recurrent cerebrovascular events in patients with symptomatic intracranial atherosclerosis?
Background. Intracranial atherosclerosis is one of the leading causes of ischemic stroke worldwide, especially among people of Asian, African, and Hispanic descent (Gorelick et al., 2008; Qureshi and Caplan, 2014; Ritz et al., 2014). In China and other Asian countries, intracranial atherosclerotic stenosis accounts for 33% to 67% of strokes or transient ischemic strokes (Huang et al., 1997; Wong et al., 2000; Lee et al., 2003; De Silva et al., 2007). Even worse, intracranial atherosclerotic stenosis is also associated with an increased risk of recurrent stroke (Gorelick et al., 2008). Currently, the optimal management of intracranial atherosclerosis is based on a combination of antiplatelet drugs and cerebrovascular risk factor controls (Kernan et al., 2014). However, the annual risk of recurrent ischemic stroke and transient ischemic attack is still unacceptably high, ranging from 6% to 18%, depending on the patient population (Chimowitz et al., 2005; Chimowitz et al., 2011; Wang et al., 2013; Amarenco et al., 2016; Park et al., 2017). Thus, the management of patients with intracranial atherosclerosis is still far from satisfactory, and new therapies are urgently needed to reduce recurrent strokes and improve patient prognosis.
Summary of Evidence. The search strategy commissioned to answer this question uncovered a systematic review, a small pilot prospective cohort study, and two small RCTs (Table 1). Li et al. (2015) recruited 10 patients with unilateral middle cerebral artery stenosis and 24 healthy volunteers to assess the safety and feasibility of RIC in patients with intracranial atherosclerosis. RIC was safe and well tolerated in patients with intracranial atherosclerosis, and it exerted no significant influence on heart rate, oxygenation index, or mean flow velocity.
Meng et al. (2012) conducted two RCTs to assess the benefits of RIC in intracranial atherosclerosis patients who were at or below 80 years of age, or above 80, respectively. In one RCT, 103 patients aged 18 -80 years who had suffered a stroke or transient ischemic attack within the previous 30 days were recruited and allocated to two groups (intervention = 51 patients; control = 52 patients) (Meng et al., 2012). Patients in the intervention group underwent additional RIC twice daily for 300 consecutive days. Sixty-eight patients completed the study, and the incidences of recurrent stroke at 90 and 300 days were only 5% and 7.9% in the RIC group (n = 38), but 23.3% and 26.7% in the control group (n = 30). Importantly, RIC also improved recovery from the initial stroke, as measured by 90-day mRS scores of 0-1 (65.8% versus 13.3%, p < 0.001). The ratios of improved cerebral perfusion, as measured by single photon emission computed tomography, were 31.6% versus 6.7% at day 90 (p = 0.012) and 76.3% versus 53.3% at day 300 (p < 0.01).
In the second RCT study, Meng and colleagues (2012) recruited 79 patients aged 80-95 years old with ischemic stroke or TIA within the previous 7 days (intervention = 40; control = 39). Patients received either RIC or sham RIC for 180 consecutive days. Fifty-eight patients completed the study (30 in the RIC group and 28 in the sham group), and the recurrence of stroke and transient ischemic attack was 30% versus 67.8%, respectively (log-rank test, p = 0.004). Additionally, on day 180, the NIHSS scores were 2.97 ± 1.97 versus 4.82 ± 2.72 (p < 0.01) and the mRS scores were 1.4 ± 1.0 versus 2.3 ± 1.1 (p<0.01) in RIC versus sham groups.
The meta-analysis of RIC for ischemic stroke included the two aforementioned RCTs as well as studies of patients with intracranial atherosclerosis (Zhao et al., 2018b). The results revealed that recurrent stroke is significantly reduced by RIC in patients with symptomatic intracerebral atherosclerosis (RR 0.32, 95% CI 0.12 to 0.83).
Panel Recommendations.
In patients with symptomatic intracranial atherosclerosis, long-term repeated RIC can be used to prevent recurrent ischemic cerebrovascular events (conditional recommendation, low confidence in estimate of effects; see E-Table A2 and E-Table B2 in the Online Supplement).
Discussion
Justification. This recommendation was based mainly on two RCTs and the meta-analysis of their results. The two RCTs demonstrated that repeated RIC, applied consecutively after ischemic cerebrovascular events, could not only prevent the recurrence of ischemic cerebrovascular events, but also facilitate the recovery of neurological deficits. In addition, RIC is not only effective in patients aged 18 to 80 years, but also effective in elderly patients aged >80 years. Thus, if patients experience ischemic cerebrovascular events caused by intracranial atherosclerosis, RIC should be considered as a strategy to prevent recurrent cerebrovascular events and facilitate the recovery of the index neurological deficits.
Implementation considerations. As recurrent ischemic cerebrovascular events were much more frequent in the several days immediately after the index ischemic events (Wang et al., 2013; Banerjee and Chimowitz, 2017), it is advisable to apply RIC as early as possible after the index event (within hours or days of the event). More importantly, repeated RIC should be applied consecutively for 180 to 300 days, or much longer.
Future studies. Although previous studies have demonstrated the safety and efficacy of RIC for patients with intracranial atherosclerosis, these studies recruited only a small number of patients and suffer from multiple methodological limitations (including per-protocol analyses and large numbers of patients lost during follow up). Therefore, much more powerful studies with large sample sizes are urgently needed to verify these important results. In addition, all participants recruited to clinical trials appear to display good compliance to the RIC regimen, although the daily use of RIC may not be executed successfully for all patients in real clinical practice. Therefore, it is worth investigating whether the use of RIC every 3 days, 7 days, or much longer periods is also effective.
Clinical question IV: Should RIC be used to improve neurological outcomes in patients with cerebral small vessel disease?
Background. Cerebral small vessel disease is responsible for approximately 25% of all ischemic strokes. It causes damage to deep cerebral grey and white matter, which can be detected using brain imaging as leukoaraiosis, small subcortical infarcts, and microbleeds (Bamford et al., 1987; Petty et al., 2000; Wardlaw et al., 2013a; Wardlaw et al., 2013b; Zwanenburg and van Osch, 2017). More than half of all elderly people display cerebral leukoaraiosis, as it is estimated to range from 70-80% in people over 60 years (Liao et al., 1997; de Leeuw et al., 2001; Schmidt et al., 2011). Although strokes caused by cerebral small vascular disease are less severe in terms of their clinical outcome during the acute phase and short-term prognosis (Sacco et al., 1991; Kolominsky-Rabas et al., 2001), the long-term outcome of these patients is not benign, and is associated with functional impairments, cognitive decline, and mortality (Norrving, 2008; Pinter et al., 2015).
Summary of Evidence. The search strategy for this question yielded two small RCTs encompassing 53 participants (Table 1). Both studies compared one year of RIC with sham RIC. One study recruited 17 patients with cerebral small vascular disease and randomly allocated them to the RIC group (n = 9) or sham group (n = 8) (Mi et al., 2016). Patients received medical management and RIC or sham RIC twice daily for one year. The mean flow velocity of the middle cerebral artery (evaluated by transcranial Doppler) was accelerated (57.33 [52.33-61.34] versus 51.33 [48.83-58.33], p = 0.038) and the post-treatment volume of the white matter lesions (evaluated by MRI) was reduced (4.19 [2.96-7.25] versus 6.06 [4.67-10.95], p = 0.050) after one year of RIC treatment, but there was no statistically significant difference between the two groups (p > 0.05 each). Another RCT assessed RIC in patients with cerebral small vessel disease-related mild cognitive impairment (Wang et al., 2017b). Thirty-six patients were recruited and randomly allocated to the RIC (n = 18) or sham group (n = 18). After one year of treatment, the white matter hyperintensity volume on MRI was significantly reduced by RIC compared to sham treatment (-2.632 ml versus -0.935 ml, P = 0.049) and visuospatial and executive abilities were also significantly improved (0.639 versus 0.191; P = 0.048). Changes of the pulsation indices of the middle cerebral arteries (evaluated by transcranial Doppler) from baseline to one year were significantly different between the two groups of patients (right side: -0.075 versus 0.043; P = 0.030; left side: -0.085 versus 0.043; P = 0.010).
Panel Recommendations.
In patients with cerebral small vessel disease and those who suffer from cerebral small vessel disease-related cognitive impairment, there is insufficient data to recommend routine use of RIC as an adjunctive therapy (conditional recommendation, very low confidence in estimate of effects; E-Table A3 and E-Table B3).
Discussion
Justification and implementation. These recommendations are based on two single-center RCTs that recruited a very small number of patients and in which the overall results for RIC in terms of cognitive function are neutral. Furthermore, the real benefits of RIC for patients with cerebral small vessel disease might be concealed due to the low power of the clinical study (e.g., small sample size) and large percentage of patients lost during follow up. As such further large adequately powered randomized controlled trials are needed.
Future studies.
Additional studies should be conducted to validate the published findings. Many key variables that could impact the results (including the management of blood pressure, glucose, and lipids) should be addressed rigorously in future studies. Additionally, it would be worth assessing the benefits of RIC for patients with vascular cognitive impairment, quite possibly the most common form of cognitive dysfunction (Roman et al., 2004). “Vascular dementia” in its different forms shows times of stable cognitive function and sometimes even remissions, which is strikingly different to AD. Longer phases of stable cognitive function, and more frequent and more pronounced remissive phases could be a relevant endpoint in future studies as this is something one might expect from RIC.
Clinical question V: Should RIC be used to reduce perioperative complications in patients with carotid artery stenosis who are treated with carotid stenting?
Background. Currently, carotid artery stenting is one of the most common revascularization procedures to prevent ischemic cerebrovascular events (Benjamin et al., 2017). Several large-scale trials have determined the benefits of carotid stenting among patients with recent ischemic events as well as patients who are asymptomatic (Barnett et al., 1991; Hobson et al., 1993; Walker et al., 1995; European Carotid Trialists' Collaborative Group, 1998; Halliday et al., 2004; Bonati et al., 2015; Rosenfield et al., 2016). However, perioperative complications cannot be completely avoided. The rate of combined 30-day stroke and death ranges from 6% to 9% among symptomatic patients and from 2% to 4% among asymptomatic patients (Mas et al., 2006; Ringleb et al., 2006; Algra et al., 2010). In addition, previous studies found new cerebral ischemic lesions on post-procedure MRI images in 20% to 70% of subjects undergoing carotid stenting (Heider et al., 2007; Kim et al., 2007; Lacroix et al., 2007; Bonati et al., 2010; Bijuklic et al., 2013). To make matters worse, these subclinical embolisms may have adverse effects on long-term cognitive function (Corea et al., 2001; Zhou et al., 2017).
Summary of Evidence. The search strategy for this question uncovered one RCT (Table 1) (Zhao et al., 2017b). The study recruited 189 patients with severe carotid artery atherosclerotic stenosis (70% - 99%) and allocated them to three groups (RIC, sham, and control groups). All patients received standard medical management, and patients in the treatment group received RIC twice daily for 2 weeks before the carotid stenting procedure. Finally, 162 patients completed carotid stenting and post-treatment MRI, and the incidence of new MRI brain lesions in the RIC group was 15.87%, lower than in the control group (41.27%; relative risk, 0.39; 96% confidence interval, 0.21 – 0.82; p = 0.002) and the sham group (36.51%; relative risk, 0.44; 96% confidence interval, 0.20 – 0.91; p = 0.008). In addition, the average infarct volume was 0.03 mL (0.02 - 0.05), which was also significantly smaller than the 0.08 mL (0.06-0.12) in the sham group and 0.07 mL (0.05-0.10) in the control group (p < 0.001 for each).
Panel Recommendations
In patients with carotid artery stenosis who are treated with carotid stenting, two weeks of RIC treatment before the operation may be considered as a strategy to prevent perioperative complications (conditional recommendation, moderate confidence in estimate of effects; E-Table A4 and E-Table B4).
Discussion
Justification and implementation. The RCT eligible for making this recommendation reported that two weeks of RIC before operation could significantly reduce the incidence of posttreatment brain lesion and infarct volume, and that the incidence of ischemic cerebrovascular events tends to be lower in the RIC group. Although RIC is associated with much higher rates of arm skin petechiae from repeated cuff applications, the adverse events are not serious and exert no harmful influence. Therefore, we suggest that two weeks of repeated RIC before the operation may be considered to reduce the perioperative complications for patients who will undergo carotid stenting.
Future studies.
Much larger studies are needed to assess the benefits of RIC in preventing ischemic cerebrovascular events after carotid stenting. Furthermore, as the silent infarcts caused by microemboli have potential adverse effects on long-term cognitive function (Corea et al., 2001; Zhou et al., 2017), future studies should include a battery of long-term cognitive and psychological function tests in their outcome assessments. Additionally, postprocedural silent cerebral ischemic lesions are not an uncommon complication in many endovascular or vascular surgeries; therefore, it is also worth assessing whether RIC prevents silent cerebral embolisms during other medical procedures.
Clinical question VI: Can RIC be performed safely to improve functional outcomes in patients with intracranial hemorrhage?
Background. Intracranial hemorrhage, a devastating disorder with poor prognosis and high mortality, can be classified into intracerebral hemorrhage, subarachnoid hemorrhage, and epidural and subdural hemorrhages (Naidech, 2011). Subarachnoid hemorrhage and intracerebral hemorrhage are life-threatening conditions and represent the major, least-treatable subtypes. Subarachnoid hemorrhage has a high rate of death and complications, and 80% of cases are caused by the rupture of an intracranial aneurysm (van Gijn and Rinkel, 2001; Long et al., 2017). The average mortality for subarachnoid hemorrhage is ~51%, while 46% of survivors may suffer long-term cognitive impairments (Hop et al., 1997; Hackett and Anderson, 2000; Mayer et al., 2002). Although coil embolization and open surgery can be used to prevent the re-rupture of aneurysms, few therapies are available for the treatment of subarachnoid hemorrhage (Connolly et al., 2012). Intracerebral hemorrhage also has a poor prognosis; its 30-day mortality ranges from 35 to 52% and its one-year functional independence rate ranges from 17 to 25% (van Asch et al., 2010; Poon et al., 2014; Moulin and Cordonnier, 2015). Unfortunately, clinical trials have failed to demonstrate the superiority of surgical hematoma evacuation and stereotactic or endoscopic clot aspiration over medical management (Mendelow et al., 2005; Gregson et al., 2012). Thus, it remains an intractable condition and the least treatable form of stroke.
Summary of Evidence. The search strategy for this question found three studies on subarachnoid hemorrhage, including one Phase Ib study, one Phase I study, and one matched cohort study (Table 1). The Phase Ib study assessed the safety and feasibility of increasing durations of limb ischemia in patients with subarachnoid hemorrhage after endovascular coiling or surgical clipping therapy (Koch et al., 2011). In both of the lead-in and dose escalation phases, preconditioning procedures were well tolerated. No session was prematurely terminated due to subject discomfort, and no objective signs of neurovascular injury were observed. Another Phase I study assessed the safety and feasibility of lower-limb RIC for subarachnoid hemorrhage patients who were secured by endovascular coiling or surgical clipping (Gonzalez et al., 2014). The results showed that no patient developed deep venous thrombosis or injury, and no patients developed delayed ischemic neurological deficits. A matched cohort study, consisting of 21 RIC patients and 61 matched controls, aimed to assess the potential benefits of lower-limb RIC for patients with aneurysmal subarachnoid hemorrhages (Laiwalla et al., 2016). The latter study found that RIC was independently associated with good outcomes defined as mRS of 0 to 2 (odd ratio 5.17; 95% confidence interval 1.21 – 25.02). In addition, RIC elicited a trend toward lower incidences of stroke (28.6 versus 47.5 %) and death (4.8 versus 19.7 %).
Panel Recommendations
In patients with aneurysm subarachnoid hemorrhage who have been treated with endovascular coiling or surgical clipping, there is insufficient evidence to recommend routine use of RIC as an adjunctive therapy (conditional recommendation, very low confidence in estimate of effects; E-Table A5 and E-Table B5).
Discussion
Justification. Currently there are only two case series and one matched cohort study, supporting a role of RIC in patients with aneurysm subarachnoid hemorrhage after endovascular coiling or surgical clipping, thus a RCT demonstrating efficacy is therefore needed.
Future study. More recently, preclinical studies have found that repeated RIC accelerates hematoma resolution and improves neurological outcomes after intracerebral hemorrhage (Geng et al., 2012; Vaibhav et al., 2018). As subarachnoid and intracerebral hemorrhages share many common pathophysiological mechanisms, the safety, feasibility, and efficacy of RIC in patients with intracerebral hemorrhage needs to be investigated as soon as possible. Furthermore, a carefully conducted RCT is needed to determine whether RIC is effective in this setting.
Clinical question VII: Which cohorts of patients with cerebrovascular disease qualify for RIC treatment?
Background. Cerebrovascular diseases are divisible into ischemic and hemorrhagic cerebrovascular diseases, each of which also has several subtypes. Although the therapeutic treatments of each subtype have significantly improved during the past several decades, they are still unsatisfactory. Therefore, RIC has been investigated as an adjunctive therapy (i.e., both prevention and treatment) for improving the outcomes of cerebrovascular disease.
Summary of Evidence. The answers to this clinical question derive from the aforementioned historical studies (Table 1) and one systematic review that specifically investigated RIC for the management of ischemic stroke. Two pilot RCTs assessed the safety and preliminary efficacy of RIC in patients with acute ischemic stroke who did not receive thrombolysis or thrombectomy (England et al., 2017; Li et al., 2018). One prospective study specifically assessed the safety and feasibility of RIC in acute ischemic stroke patients with large artery occlusions treated with thrombectomy (Zhao et al., 2018c). One RCT specifically assessed the safety and efficacy of prehospital RIC in the treatment of acute ischemic stroke patients who were also candidates for intravenous thrombolysis (Hougaard et al., 2014). One recently completed trials assessed RIC in patients treated with intravenous thrombolysis or endovascular thrombectomy. One RCT assessed the safety and efficacy of pre-procedural RIC for the treatment of severe carotid artery stenosis patients who were treated with carotid stenting (Zhao et al., 2017b). Two small RCTs assessed the efficacy of long-term RIC for the treatment of cerebral small vessel disease (Mi et al., 2016; Wang et al., 2017b). One observational study assessed the feasibility and safety of RIC in patients with unilateral middle cerebral artery stenosis and compared the data to healthy volunteers. Thereafter, two RCTs specifically assessed the efficacy of RIC in patients with symptomatic intracranial atherosclerosis (Meng et al., 2012; Li et al., 2015; Meng et al., 2015). One Phase I study and one Phase Ib study assessed the safety and feasibility of RIC in patients with aneurysm subarachnoid hemorrhage, after coiling or surgical clipping of the aneurysm (Koch et al., 2011; Gonzalez et al., 2014), and another matched cohort study evaluated the efficacy of RIC in this patient population (Laiwalla et al., 2016).
Several study protocols have been published: one ongoing study is investigating RIC in patients with Moyamoya disease (Li et al., 2017), while another ongoing study is investigating RIC in patients with minor ischemic stroke or transient ischemia attack (Liu et al., 2018). A third multicenter trial is currently investigating RIC in patients with symptomatic intracranial atherosclerosis (Hou et al., 2016). Besides the aforementioned published studies and study protocols, the prevention of ischemic stroke with RIC was assessed non-specifically for the protection of other organs during operations, such as percutaneous coronary intervention (Hoole et al., 2009; Davies et al., 2013; Sloth et al., 2014; Elbadawi et al., 2017), coronary artery bypass grafting (Hausenloy et al., 2015; Meybohm et al., 2015; Benstoem et al., 2017), and cardiac surgery and peripheral vascular surgery (Desai et al., 2011; Twine et al., 2014; Zarbock et al., 2015; Garcia et al., 2016; Lotfi et al., 2016; Cho et al., 2017; Kahlert et al., 2017; Pierce et al., 2017).
Panel Recommendations. This section simply lists a narrative review of the application of RIC for cerebrovascular disease management as a paucity of research data in this area precludes the development of meaningful recommendations at this time.
Discussion. RIC has been widely investigated in clinical studies for the prevention and treatment of cerebrovascular disease, particularly the ischemic type. Clinical studies have demonstrated the benefits of RIC in patients with acute ischemic stroke, intracranial atherosclerosis, cerebral small vessel disease, and carotid artery stenosis patients treated with stenting. During some surgeries, the prevention of ischemic stroke by RIC was also investigated non-specifically. Although RIC has been investigated in patients with intracranial hemorrhage, only the aneurysm subarachnoid hemorrhage subtype was scrutinized. Thus, the safety and efficacy of RIC in patients with intracerebral hemorrhage urgently deserves to be investigated. Recently, repeated RIC was proposed to mimic the effects of regular exercise in healthy individuals (Zhao et al., 2018a). Due to its cost effectiveness, ease of use, and good safety profile, it would be worthwhile to explore the effects of RIC in preventing initial strokes, as well as the potential benefits of long-term repeated RIC treatment for cerebrovascular disease management. Additionally, investigating long-term repeated RIC in all-comer patients with cerebrovascular diseases would be an excellent new research goal.
Clinical question VIII: Which specific RIC protocols should be used in patients with cerebral vascular disease?
Background. RIC has been widely investigated in patients with cerebrovascular disease, but different studies used distinct RIC protocols (Zhao et al., 2019). The most commonly used protocols in clinical studies are four cycles of unilateral arm ischemia or five cycles of bilateral arm ischemia for 5 minutes, each followed by 5 minutes of reperfusion. The stimulus sites for RIC include the thigh and arm, and the duration of RIC treatment varies from once to twice daily for 12 months.
Summary of Evidence. The answers to this clinical question derive from previously published studies. The detailed protocols of RIC used in different studies are summarized in Table 1. Protocols of 4 × 5 minute inflations/deflations of the cuff on one arm were used in four studies of acute ischemic stroke; 5 × 5 minutes of cuff inflations/deflations on bilateral arms were used in six studies (all these studies were conducted by the same study group from Xuanwu Hospital); and 4 × 5 minutes of cuff inflations/deflations on one leg were used in two studies of aneurysm subarachnoid hemorrhage. A cuff pressure of 200 mmHg was most commonly used in previous studies, or 20 and 25 mmHg (or much higher) above systolic pressure, until the pulse was abolished. The frequency of RIC varied most—ranging from a single episode, once daily for 7 and 14 days, and twice daily for 2 weeks, 180 days, 300 days, or 1 year.
Recommendations. For patients with cerebrovascular disease, we suggest selecting RIC protocols according to the protocols used in similar previous studies or their modifications. We also suggest that the arm(s) be used as the RIC stimulus site (conditional recommendation, panel experts’ consensus).
Discussion
Justification and implementation. No study specifically investigated RIC protocols in different patients with cerebrovascular disease. This recommendation was made based on a consensus of expert opinion, accounting for clinical experiences, and completed clinical trials. We recommend that the selection of RIC protocols be based on those previously employed in successful clinical trials. In addition, as RIC is a non-invasive physical therapy and no serious adverse events have been reported, modifications of current RIC protocols (e.g., duration or number of RIC episodes) according to specific conditions are also worth considering. Although there may be no differences in RIC stimulation of the arm or leg, RIC of the leg may need much higher cuff pressure, cause more serious discomfort, and perhaps lead to deep venous thrombosis. Therefore, we recommend the arm(s) as a superior RIC stimulus site.
Future studies.
Current RIC protocols derive from the first experiment on ischemic preconditioning conducted over 30 years ago (Murry et al., 1986); however, the optimal RIC protocol is still undefined. Therefore, future studies should be performed to explore the optimal cycles, duration of ischemia, site of stimulus, and length of RIC for various types of cerebral vascular diseases.
Clinical question IX: What are the contradictions of RIC, and which parameters should be scrutinized to ensure the safety of RIC?
Background. As the limbs are easy to access even in intensive care wards and tolerate ischemia well, RIC is generally initiated in the limbs with several cycles of brief ischemia followed by reperfusion. Ischemia is usually initiated by a blood pressure cuff, so that the procedures are similar to blood pressure measurements, with the exception of the duration of the ischemia. Although RIC is relatively safe, a physical examination should be performed before the RIC procedure, and several contradictions (including soft tissue injury and orthopedic injury in the limbs, blood pressure >200 mm Hg, vessel occlusive comorbidities or venous thrombosis in limbs, etc.) may prevent its application.
Summary of Evidence. The answers to this clinical question derive from the completed clinical studies. Meng et al. (2012; 2015) excluded patients with any soft tissue, orthopedic, and vascular injury of the extremities; those with hematologic disease and whose systolic blood pressure >200mmHg were also excluded. In another study, Meng et al. (2012; 2015) also excluded those with peripheral blood vessel disease (especially subclavian arterial stenosis). In Li’s study, healthy volunteers underwent psychiatric and medical evaluations, including physical examinations, body weight index measurements, blood tests, and electrocardiograms (Li et al., 2015). In patients with subarachnoid hemorrhage, Koch’s study excluded patients with a history of peripheral vascular disease and ankle brachial index <0.7, and those with any extremity soft tissue, orthopedic, or vascular injury, such as superficial wounds, cellulitis, fracture, deep vein thrombosis (Koch et al., 2011); those with a history of deep vein thrombosis, peripheral vascular disease, peripheral neuropathy were also excluded, as were those patients with new findings of these diseases upon physical examination (Gonzalez et al., 2014). In Wang’s study, patients with significant bleeding disorders, systolic blood pressure >200 mm Hg with medication, any soft tissue or vascular injury, and any disease of the extremities that would contraindicate RIC were excluded (Wang et al., 2017b). Mi’s study also excluded patients with coagulative dysfunction (Mi et al., 2016). Zhao’s study excluded patients with uncontrolled hypertension (defined as systolic blood pressure ≥200 mm Hg despite medication usage at enrollment), any vascular, soft tissue, or orthopedic injury (e.g., superficial wounds and fractures of the arm), and peripheral vascular disease (especially subclavian arterial and upper limb artery stenosis or occlusion). In patients with acute ischemic stroke (including those treated with intravenous thrombolysis and endovascular therapy), no specific evaluation was performed.
Panel Recommendations.
For patients who are candidates for RIC, we recommend that a physical examination and vascular ultrasound examination of the limb should be performed before any RIC procedures are initiated (conditional recommendation, panel experts’ consensus), provided the necessary equipment is available. Alternatively, stricter exclusion criteria are recommended if there are safety concerns that have not been clarified. In patients with traumatic or ischemic injuries and dysmelia that may hamper the procedures on the initiation tissue, we recommend that RIC not be used (conditional recommendation, panel experts’ consensus)
Discussion
Justification. In the historical studies, no clinical examination before RIC procedures was put forward. The recommendations for this question were made based on a consensus of expert opinion according to exclusion criteria of the historical studies and clinical experiences.
Implementation. We recommend that a physical examination should be performed for all RIC candidates. The physical examination should include measurements of blood pressure and bilateral radial and dorsalis pedis pulse, examinations of skin and soft tissue injuries of limbs, and identification of any dysmelia or any other abnormal conditions. The goals of the physical examination are to exclude those with any soft tissue injury, orthopedic injury in the limbs, and patients with blood pressure >200 mm Hg despite medication usage. Vascular ultrasound should be performed to detect any vessel occlusive comorbidities or venous thrombosis. Furthermore, in patients with a high risk of arterial occlusion or stenosis and with limb ischemia, the inspection should encompass venous thrombosis, arterial stenosis, plaques, and vessel abnormalities.
Clinical question X: What equipment should be chosen to perform the RIC procedures?
Background. The potential clinical value of RIC became clear after the discovery of the phenomenon by Przyklenk and her colleagues (Przyklenk et al., 1993). Since that publication, many clinical studies have been conducted using a variety of methods to induce RIC, including manual RIC with sphygmomanometers and automated devices.
Summary of Evidence. The evidence consulted to answer this clinical question derives from historical clinical studies, and the methods used to induce RIC and its protocols are summarized in Table 1. The Xuanwu Hospital study group used an automated device (developed by Beijing Renqiao Cardio-Cerebrovascular Disease Prevention and Control Institution) to induce unilateral or bilateral arm RIC; both RIC and sham RIC devices were used in several of their studies (Meng et al., 2012; Hess et al., 2015; Li et al., 2015; Meng et al., 2015; Mi et al., 2016; Wang et al., 2017b; Zhao et al., 2017b). In Li and England’s study of acute ischemic stroke patients, manual RIC was induced by using a standard upper arm blood pressure cuff (England et al., 2017). In Hougaard’s study, RIC was induced by using an automated device produced by CellAegis Devices Incorporated (Hougaard et al., 2014). In patients with subarachnoid hemorrhage, the authors also manually induced RIC with a standard blood pressure cuff (Koch et al., 2011; Gonzalez et al., 2014).
Panel Recommendations.
We recommend that RIC procedures be performed by using automated devices or manual blood pressure cuffs (conditional recommendation, panel experts’ consensus).
Discussion
Justification. Manual RIC using a standard blood pressure cuff placed on arms or legs and inflated to 200 mmHg (or some degree of mmHg above systolic pressure) may be the simplest prototype of the RIC method. Currently, several companies have developed automated devices that are convenient for RIC applications. Theoretically, if done correctly, the effects of manual RIC should be the same as that induced by automatic devices.
Implementation. In clinical practice, manual RIC is both impractical and costly, as it requires a dedicated individual to ensure that a specific algorithm of inflation/deflation cycles is applied over a treatment period of 40 minutes or more. An automated system is preferable, particularly for high-acuity populations (e.g., patients with acute ischemic stroke) who typically receive many treatments in several physical locations (ambulance, emergency department, and catheterization laboratory) over a very short period.
Future study. At least four start-up companies have undertaken the development of an automated RIC device (Garratt and Leschinsky, 2017). Two of the devices were used in patients with cerebrovascular disease. CellAegis Device, Inc, a Canadian company, developed a device, called autoRIC, which received market approval in Europe in 2012 and in Canada in 2013 for the treatment of patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. In China, a device called DoctorMate or XuanyiTong has been developed by Beijing Renqiao Cardio-Cerebrovascular Disease Prevention and Control Institution. This device has been approved by the Chinese FDA, and received market approval in China in 2016 for the management of cerebrovascular disease. In the future, different automated RIC devices should be developed for distinct clinical settings, such as the ambulance, emergency department, operation room, ward, and home. For the application of chronic RIC, it would be important if the automated devices could come with compliance monitoring of the intervention.
Clinical question XI: What adverse events occur in the aftermath of RIC?
Background. Distinct from pharmacotherapies, RIC is a physical therapy and can be induced with a standard blood pressure cuff. Therefore, RIC is less likely to interact with medications and impact the proper function of the liver and kidneys. However, the persistent occlusion of unilateral or bilateral limbs’ arteries for 5 minutes may be uncomfortable and influence hemodynamics, and repeated occlusion and reperfusion of arteries may also cause injuries to local tissue in the limbs.
Summary of Evidence. The answers to this clinical question derive from the historical clinical studies. Li’s study assessed the safety of RIC in healthy volunteers and patients with intracranial atherosclerosis; in both patients and heathy volunteers, RIC was well tolerated without any complications, and it had no significant influence on the tissue oxygenation index of the ischemic forearm and brain, or the mean flow velocity of the middle cerebral arteries (Li et al., 2015). However, in healthy volunteers, there was a significant reduction in diastolic blood pressure (73.4 ± 7.6 mmHg versus 68.3 ± 8.2mmHg; p = 0.031) and heart rate (73.5 ± 8.3 bpm versus 68.4 ± 9.1 bpm; p = 0.027) between baseline and 30 minutes after completion of RIC procedures. In Meng’s study of patients with intracranial atherosclerosis, no local skin or vessel lesions were reported during the 300-day-long repeated pressure cuff applications (Meng et al., 2012). In Meng’s other study of RIC in octo- and nonagenarian patients, 16.7% complained of mild discomfort, but they tolerated the treatment. Transient sporadic petechiaes were observed in 3 cases during the first 30 days of the study (Meng et al., 2015). No ecchymosis, tenderness to palpation, edema, skin breakage, or other skin lesions was observed. No deep vein thromboses were detected by vascular sonography during or at the end of the 180 days of RIC.
In Hougaard’s study of prehospital RIC, no RIC-related adverse events were reported (Hougaard et al., 2014). In England’s study of acute ischemic stroke patients within 24 hours of ictus, there were no procedure-related serious adverse events and RIC did not significantly affect central blood pressure, mean arterial pressure, arterial compliance, Buckberg index, or the ipsilateral middle cerebral artery blood flow (England et al., 2017). In Li’s study of acute ischemic stroke patients within 72 hours of ictus, one patient displayed small subcutaneous bleeding at the cuff contact site, but no significant changes in blood pressure, mean velocity or pulsatility, and heart rate during repetitive RIC (Li et al., 2018). In patients with acute ischemic stroke treated with thrombectomy, Zhao et al. (2018c) found that no patient experienced serious RIC-related adverse events, and the intracranial pressure, cranial perfusion pressure, mean arterial pressure, heart rate, middle cerebral artery peak systolic flow velocity, and pulsatility index did not change significantly before, during, or after limb ischemia.
In Koch’s study of patients with subarachnoid hemorrhage, no objective signs of neurovascular injury were observed, and no subjects experienced skin breakdown, prolonged discoloration, or temperature or pulse disparities in the treated limb (Koch et al., 2011). Although deep vein thrombosis was found in three patients, only two were symptomatic, and included one in the RIC-undergoing arm and the other in the leg contralateral to the conditioned leg. Importantly, the author concluded that these were unrelated to the RIC intervention. In Gonzalez’s study of patients with subarachnoid hemorrhage, no patient developed symptomatic deep vein thrombosis, bruising, or injury related to the RIC procedure, and analyses of vital signs and additional monitoring data did not reveal any statistically significant change for any of the repeated measures analysis of variance treatments (Gonzalez et al., 2014).
In patients treated with carotid stenting, Zhao reported that the RIC procedure was completed with a high compliance rate (98.41%), but that six subjects experienced arm skin petechiae from repeated pressure cuff applications (Zhao et al., 2017b). No ecchymosis, tenderness to palpation, edema, skin breakage, or other skin lesions was observed. In patients with cerebral small vascular disease, Mi and Wang did not report any adverse events after RIC (Mi et al., 2016; Wang et al., 2017b).
Panel Recommendations. This section is only a narrative review of the clinical use of RIC for cerebrovascular disease management; therefore, no recommendations are needed.
Discussion
In patients with cerebrovascular disease, RIC was well tolerated, even when applied repeatedly for 1 year or in the in critically ill patient population. No RIC-related severe adverse events were reported. The most common adverse event was skin petechiae or light subcutaneous bleeding at the cuff contact site or the ischemic forearm; these adverse events do not seem to cause any harmful impacts and disappear within one or two weeks. Several studies have evaluated the influence of RIC on patients’ hemodynamics by using different methods in distinct patient population, and all the results are in agreement that RIC does not influence blood pressure, intracranial pressure, intracranial perfusion pressure, blood flow velocity of middle cerebral artery, or other parameters of hemodynamics. Therefore, RIC is distinct from external counterpulsation, which changes hemodynamics (Manchanda and Soran, 2007; Yang and Wu, 2013). Rather, RIC exerts its protective roles through other mechanisms, and has no significant influence on cerebral hemodynamics. However, one study of healthy volunteers found a significant reduction in diastolic blood pressure and heart rate after RIC. No other studies reported this phenomenon; thus, further investigations are needed to determine whether this phenomenon was caused by bias or by RIC itself. It is important to note that a Cochrane systematic review of RIC for preventing and treating ischemic stroke reported no severe adverse events related to RIC procedures (Zhao et al., 2018b). In patients with subarachnoid hemorrhage, one study reported that deep vein thrombosis was a concern. Although the authors asserted that this adverse event was unrelated to the RIC intervention, in high-risk patients of deep vein thrombosis, cautions should be exercised when using RIC.
Summary
These clinical practice guidelines represent our collective synthesis of the latest evidence on the utility of RIC for cerebrovascular disease management. The guidelines offer a number of clinical recommendations pertaining to each question posed by the panel (Figure 1). Although five recommendations were prepared based on previously published studies with rigorous methodologies and three recommendations were based on the consensus of expert opinion, it must be noted that some of the latter recommendations were based on a small number of low-power clinical studies. Thus, future higher-quality studies may shift the recommendations.
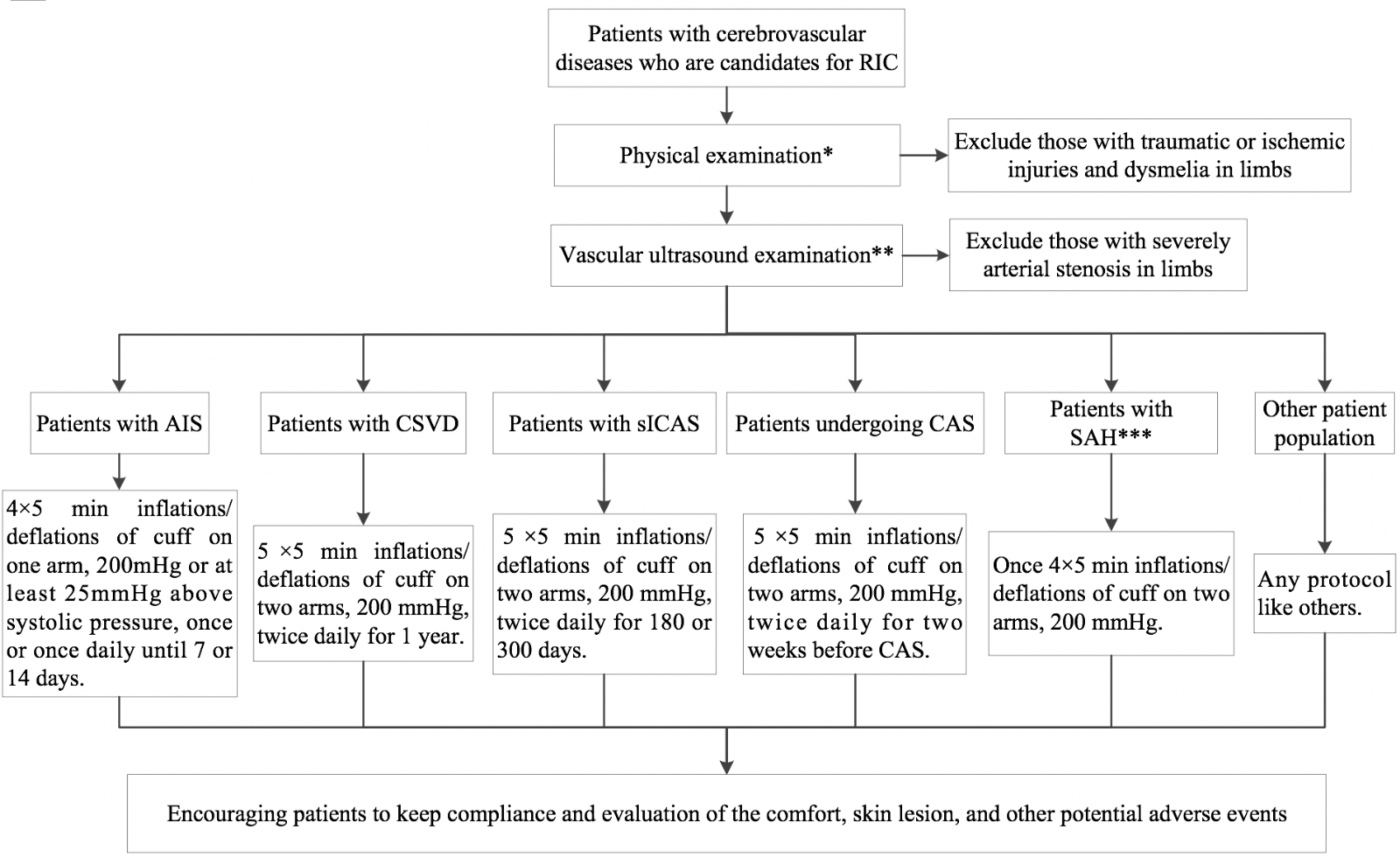
In a new window | Download PPT
Figure 1: Management of cerebrovascular disease patients with remote ischemic conditioning. RIC, remote ischemic conditioning; AIS, acute ischemic stroke; CSVD, cerebral small vessel disease; sICAS, symptomatic intracranial atherosclerosis; CAS, carotid artery stenting; SAH, subarachnoid hemorrhage.
NOTE: RIC protocols are recommended based on current evidence, but the optimal RIC protocol remains undefined. Future studies may change the recommended RIC protocols.
*Physical examination includes measurements of blood pressure and bilateral radial and dorsalis pedis pulse, examinations of skin and soft tissue injuries of limbs, and detection of any dysmelia or any other abnormal conditions.
**Vascular ultrasound examination refers to arterial and venous vessel of limbs; the inspection should encompass venous thrombosis, arterial stenosis, plaques, and vessel abnormalities.
***Patients with SAH who will use RIC should be treated with coil embolization or open surgery.
Due to its non-invasive nature and excellent safety profile, the use of RIC offers promise in the broad clinical management of both ischemic and hemorrhagic cerebrovascular disease. However, more studies are urgently needed, including investigations of the precise underlying mechanisms and determination of optimal RIC protocols. Even more importantly, clinicians and investigators should exercise caution regarding patient discomfort, transient sporadic petechiaes, and other potential adverse events during the implementation of RIC. Finally, a battery of clinical examinations (e.g., physical examination and vascular ultrasound examination) is imperative before initiation of RIC procedures.
Funding
Xunming Ji was supported by the National Key R&D Program of China (No. 2017YFC1308400), Chang Jiang Scholars Program (No. T2014251), National Natural Science Foundation of China (81801313 and 81620108011), and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201706).
Derek Hausenloy was supported by the British Heart Foundation (FS/10/039/28270), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References

In a new window | Download PPT
Figure 1: Management of cerebrovascular disease patients with remote ischemic conditioning. RIC, remote ischemic conditioning; AIS, acute ischemic stroke; CSVD, cerebral small vessel disease; sICAS, symptomatic intracranial atherosclerosis; CAS, carotid artery stenting; SAH, subarachnoid hemorrhage.
NOTE: RIC protocols are recommended based on current evidence, but the optimal RIC protocol remains undefined. Future studies may change the recommended RIC protocols.
*Physical examination includes measurements of blood pressure and bilateral radial and dorsalis pedis pulse, examinations of skin and soft tissue injuries of limbs, and detection of any dysmelia or any other abnormal conditions.
**Vascular ultrasound examination refers to arterial and venous vessel of limbs; the inspection should encompass venous thrombosis, arterial stenosis, plaques, and vessel abnormalities.
***Patients with SAH who will use RIC should be treated with coil embolization or open surgery.
Supporting Information
Download Supporting Information (PDF)Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 11152 | 93 | 12 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA