Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Role of protease activated receptor-1 in thrombin preconditioning against thrombin-induced brain injury in mice
Time:2019-11-05
Number:7780
Xuhui Bao, Fenghui Ye, Shu Wan, Ya Hua, Richard F. Keep, Hugh J. L. Garton, Guohua Xi
Author Affiliations


- 1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Conditioning Medicine 2019. 2(5):185-191.
Abstract
While high doses of thrombin can cause brain injury, pretreatment with a low dose of thrombin (thrombin preconditioning, TPC) protects neurons and astrocytes in culture from hypoglycemic and ischemic cell death in vitro and attenuates ischemic brain injury in vivo. This study investigated the role of protease activated receptor-1 (PAR-1), a thrombin receptor, in TPC. Wild type (WT) or PAR-1 knock-out (KO) female mice brains were respectively preconditioned with 0.1 U thrombin or saline by direct injection into the right basal ganglia. Seventy-two hours later, mice received a second injection of a large injurious dose (0.5 U) of thrombin. Brain water and sodium content, blood-brain barrier disruption, neuronal death, DNA injury, and behavioral deficits were evaluated on day 1. A subgroup of mice was euthanized 72 hours after TPC to examine the expression of heat shock protein (HSP) 25, HSP 32 and ceruloplasmin by Western blotting. This study found that TPC markedly attenuated brain edema (water content (%): 79.25 ± 0.51 in the WT TPC group vs. 80.30 ± 0 .18 in the WT saline group, n = 6 each group, p < 0.01), blood-brain barrier disruption, neuronal death, DNA injury, and behavioral deficits (corner turn test scores (%): 47 ± 18 in the WT TPC group, n = 7 vs. 85 ± 24 in the WT saline group, n = 6, p < 0.01) that followed injection of a large dose of thrombin in WT mice without TPC. This effect of TPC was absent in PAR-1 KO mice. In addition, TPC upregulated brain HSP25, HSP32, and ceruloplasmin in WT mice but not PAR-1 KO mice (p < 0.01). In conclusion, PAR-1 plays a crucial role in the protective effects of TPC in female mice possibly via upregulation of heat shock proteins and ceruloplasmin.
Keywords: protease activated receptor, thrombin, preconditioning, heat shock protein, ceruloplasmin.
Abstract
While high doses of thrombin can cause brain injury, pretreatment with a low dose of thrombin (thrombin preconditioning, TPC) protects neurons and astrocytes in culture from hypoglycemic and ischemic cell death in vitro and attenuates ischemic brain injury in vivo. This study investigated the role of protease activated receptor-1 (PAR-1), a thrombin receptor, in TPC. Wild type (WT) or PAR-1 knock-out (KO) female mice brains were respectively preconditioned with 0.1 U thrombin or saline by direct injection into the right basal ganglia. Seventy-two hours later, mice received a second injection of a large injurious dose (0.5 U) of thrombin. Brain water and sodium content, blood-brain barrier disruption, neuronal death, DNA injury, and behavioral deficits were evaluated on day 1. A subgroup of mice was euthanized 72 hours after TPC to examine the expression of heat shock protein (HSP) 25, HSP 32 and ceruloplasmin by Western blotting. This study found that TPC markedly attenuated brain edema (water content (%): 79.25 ± 0.51 in the WT TPC group vs. 80.30 ± 0 .18 in the WT saline group, n = 6 each group, p < 0.01), blood-brain barrier disruption, neuronal death, DNA injury, and behavioral deficits (corner turn test scores (%): 47 ± 18 in the WT TPC group, n = 7 vs. 85 ± 24 in the WT saline group, n = 6, p < 0.01) that followed injection of a large dose of thrombin in WT mice without TPC. This effect of TPC was absent in PAR-1 KO mice. In addition, TPC upregulated brain HSP25, HSP32, and ceruloplasmin in WT mice but not PAR-1 KO mice (p < 0.01). In conclusion, PAR-1 plays a crucial role in the protective effects of TPC in female mice possibly via upregulation of heat shock proteins and ceruloplasmin.
Keywords: protease activated receptor, thrombin, preconditioning, heat shock protein, ceruloplasmin.
Introduction
While thrombin is a key enzyme of the blood coagulation system, it also plays an important role in brain injury and recovery after stroke. Thus, high concentrations of thrombin may cause brain injury and neuronal cell death (Xi et al., 2006; Krenzlin et al., 2016; Mao et al., 2016), but low concentrations can exert neuroprotective effects (Xi et al., 1999; Masada et al., 2000; Striggow et al., 2000; Jiang et al., 2002). Previously, we found that pretreatment with a low dose of thrombin attenuates the brain edema induced by thrombin, iron, or hemorrhage in rats (Hua et al., 2003), significantly reduces infarct size in a rat middle cerebral artery occlusion model (Masada et al., 2000), and reduces brain damage in a rat model of Parkinson’s disease (Cannon et al., 2006). This phenomenon has been termed thrombin preconditioning (TPC), or thrombin-induced brain tolerance.
Currently, the precise mechanisms of thrombin-induced tolerance to ischemic and hemorrhagic stroke are not fully understood (Xiang et al., 2018). Our previous work indicated that thrombin receptor activation, as well as upregulation of iron handling proteins and heat shock proteins, are involved in the induced tolerance (Xi et al., 1999; Xi et al., 2000; Yang et al., 2006; Bao et al., 2018). The first thrombin receptor, protease-activated receptor (PAR)-1, was identified in the 1990s (Vu et al., 1991) and helped to explain the direct cellular effects of thrombin on different cell types (Macfarlane et al., 2001). PAR-1 belongs to a family of PARs (PAR-1, -2, -3, and -4) where proteases activate the receptor via cleavage. Among these PARs, PAR-1 is the most widely expressed in neurons, astrocytes, oligodendroglia cells, and microglia (Weinstein et al., 1995; Ubl et al., 2000). Accumulating evidence has shown that thrombin and PARs are involved in neuroinflammatory and neurodegenerative processes in the central and peripheral nervous system (Wang and Reiser, 2003; Rohatgi et al., 2004; Rajput et al., 2014).
Prior studies indicate that preconditioning-induced neuroprotection is associated with new protein synthesis (Dirnagl et al., 2009; Yang et al., 2017). Our group has found that PAR-1 activation plays an important role in upregulating protective proteins leading to cell survival in vitro (Cheng et al., 2014; Bao et al., 2018). HSP25/27, HSP32, and ceruloplasmin are previously reported to be associated with cell survival from ischemic and hemorrhagic stress in numerous models (Xi et al., 1999; Gidday, 2006; Yang et al., 2006).
The present study evaluated the role of PAR-1 in TPC induced protection against thrombin-induced brain edema formation, BBB disruption, neurological deficits, and neuron cell death using PAR-1 KO mice. In addition, the role of PAR-1 in upregulating cytoprotective proteins was examined.
Materials and methods
Animal Preparation and Intracerebral Infusion
The protocols for these animal studies were approved by the University of Michigan Committee on the Use and Care of Animals. A total of 57 female mice 3-6 months of age were used in the study. WT C57BL/6J mice were obtained from The Jackson Laboratory and PAR-1 KO mice were from the University of Michigan Breeding Core.
For intracerebral infusions, animals were anesthetized with ketamine and xylazine (90 and 5 mg/kg, IP respectively) and a feedback controlled heating pad was used to maintain mice rectal temperature at 37°C. Mice were then positioned in a stereotaxic frame (Kopf Instrument, Tujunga, CA) and a cranial burr hole was drilled on the right coronal suture 2.5 mm lateral to the midline. Thrombin or saline (10 μL) was infused into the right basal ganglia at a rate of 1 μL/min through a 30-gauge needle (coordinates: 0.1 mm anterior, 3.5 mm ventral, and 2.5 mm lateral to bregma) with the use of a microinfusion pump (Harvard Apparatus, Inc.). The needle was removed 5 min after infusion and the skin incision sutured closed.
Experimental Groups
The study was divided into 2 parts. In the first part of the experiment, WT mice received an intracerebral injection of 10 μL saline (n = 15) or 0.1 U rat thrombin (Sigma, St. Louis, MO) in 10 μL saline (n = 15) into the right basal ganglia while PAR-1 KO mice received an intracerebral injection of 0.1 U rat thrombin (Sigma, St. Louis, MO) in 10 μL saline (n = 15). After 72 hours, all mice received a second injection of 0.5 U thrombin in 10 μL saline. Mice were decapitated 24 hours after the second injection to determine brain water and ion content. Corner turn tests were performed before injection and 24 hours after the second injection. In the second part of the experiment, WT mice received an intracerebral injection of 10 μL saline (n = 4) or 0.1 U thrombin (Sigma, St. Louise, MO) in 10 μL saline (n = 4) into the right basal ganglia while PAR-1 KO mice received an intracerebral injection of 0.1 U thrombin (Sigma) in 10 μL saline (n = 4). After 72 hours, mice were decapitated. Brains were removed and used to measure brain water content, and for Western blotting and histological analysis.
Brain Water and Sodium Content
Mice were decapitated under ketamine/xylazine (90/5 mg/kg, IP) anesthesia and the brains removed. The cerebellum was detached to serve as control and the cerebrum was then divided into 2 parts by coronally cutting approximately 2 mm posterior to the needle site with a blade. Then both parts of the cerebrum were divided into 2 hemispheres along the midline. Thus, a total of 5 samples from each brain were obtained: ipsilateral and contralateral anterior cerebrum, ipsilateral and contralateral posterior cerebrum, and cerebellum.
Brain samples were immediately weighed on an electronic analytical balance (model AE 100, Mettler Instrument Co, Columbus, OH) to obtain the wet weights. Brain samples were then dried in a gravity oven (Blue M. Electric Co) at 100°C for 24 hours to obtain the dry weight. Water content was expressed as a percentage of wet weight. The formula for calculation was ((Wet Weight-Dry Weight)/Wet Weight)*100%. The dehydrated samples were digested in 1 mL of 1 mol/L nitric acid for 1 week. Sodium content of this solution was measured with an automatic flame photometer (model IL943, Instrumentation Laboratory Inc, Bedford MA). Ion content was expressed in milliequivalents per kilogram of dehydrated brain tissue (mEq/kg dry wt).
Behavioral Testing
The corner turn test was used in this study to assess behavioral deficits (Mao et al., 2016). The mouse was allowed to proceed into a 30 degree corner. To exit the corner, the mouse could turn either to the left or the right, and this was recorded. This was repeated 10 times, with at least 30 seconds between trials, and the percentage of right turns was calculated. Only turns involving full rearing along either wall were included (i.e. ventral tucks or horizontal turns were excluded). The mice were not picked up immediately after each turn so that they did not develop an aversion for their prepotent turning response.
Western Blot Analysis
Animals were anesthetized and underwent transcardiac perfusion with 0.1 M phosphate buffered saline (PBS). Animals were then decapitated, brains removed, and a coronal brain slice (about 3 mm thick) 3 mm from the frontal pole was cut with a blade. The brain slice was divided into 2 hemispheres along the midline and each hemisphere was dissected into the cortex and basal ganglia. Thus, a total of 4 samples from each brain were obtained: ipsilateral and contralateral cortex, ipsilateral and contralateral basal ganglia. Tissues were then immersed in 0.3 mL Western sample buffer (62.5 mmol/L Tris-HCl, pH 6.8, 2.3% sodium dodecyl sulfate, 10% glycerol, and 5% β-mercaptoethanol) and sonicated for 10 seconds. Ten microliters of the sample solution was taken for protein assay (Thermo Scientific Pierce BCA Protein Assay Kit; Waltham, MA), while the rest was frozen at -80°C for Western blot analysis.
Western blot analysis was performed as described previously (Mao et al., 2016). Briefly, 30 μg protein was run on 12.5% polyacrylamide gels with a 4% stacking gel (SDS-PAGE) after boiling at 95°C for 5 min. The protein was transferred to hybond-C pure nitrocellulose membrane (Amersham; Little Chalfont, UK). The membranes were blocked in 5% Carnation nonfat dry milk in TBST (150 mmol/L NaCl, 100 mmol/L Tris base, 0.1% Tween 20, pH 7.6) buffer for 2 hours at room temperature. After they were washed in TBST buffer 3 times, membranes were probed with the following primary antibodies overnight at 4°C: goat anti-albumin antibody (1:3000; Bethyl Laboratories Inc.; Montgomery, TX), rabbit anti-HSP25 antibody (1:1000; Cell Signaling; Danvers, MA), rabbit anti-HSP32 antibody (1:2000; Assay Designs; New York, NY) and sheep anti-ceruloplasmin antibody (1:2000; Abcam; Cambridge, MA). After the membranes were washed with TBST buffer 3 times, the membranes were immunoprobed again with 1:5000 dilution of the second antibody (peroxidase-conjugated goat anti-rabbit, rabbit anti-goat or rabbit anti-sheep IgG) for 1 hour at room temperature. β-actin was used as a control. Finally, membranes were washed 3 times, and the antigen-antibody complexes were visualized with the ECL chemiluminescence system (Amersham; Little Chalfont, UK) and exposed to a Kodak X-OMAT film. The relative densities of detected protein bands were analyzed with ImageJ.
Fluoro-Jade C staining
Brain sections were air dried for at least 30 min on a slide warmer at 50°C. Slides bearing frozen cut tissue sections were first immersed in a basic alcohol solution consisting of 1% sodium hydroxide in 80% ethanol for 5 min. They were then rinsed for 2 min in 70% ethanol, for 2 min in distilled water, and then incubated in 0.06% potassium permanganate solution for 10 min. Slides were then transferred for 10 min to a 0.0001% solution of Fluoro-Jade C (Histo-Chem Inc) dissolved in 0.1% acetic acid. The slides were then rinsed through three changes of distilled water for 1 min per change. Excess water was drained onto a paper towel, and the slides were then air dried on a slide warmer at 50°C for at least 5 min. The air dried slides were then cleared in xylene for at least 1 min and coverslipped with DPX (Fluka) non-fluorescent mounting media.
Detection of DNA single-strand breaks by PANT staining
The DNA polymerase I-mediated biotin-dATP nick-translation (PANT) assay was performed on adjacent brain sections to detect DNA single-strand breaks according to the method described by Nakamura et al.(2005). Sections were permeabilized with 1% Triton X-100 for 30 min and endogenous peroxidases were quenched with 2% H2O2 for 20 min. After washing with 0.1 M PBS, sections were incubated in a moist chamber at 37°C for 90 min with PANT reaction mixture [5 mM MgCl2 (Roche Diagnostics, Indianapolis, IN), 10 mM 2-mercaptoethanol, 20 μg/ml bovine serum albumin, dGTP (Invitrogen, Carlsbad, CA), dCTP (Invitrogen, Carlsbad, CA), and dTTP (Invitrogen, Carlsbad, CA) at 30 μM each, 29 μM biotinylated dATP (Invitrogen, Carlsbad, CA), 1 μM dATP (Invitrogen), and 40 U/ml Escherichia coli DNA polymerase I (Invitrogen, Carlsbad, CA) in 0.1 M PBS (pH 7.4)]. Sections were incubated in streptavidin-horseradish peroxidase (Vetastain Elite ABC, Vector Labs, Burlingame, CA) in PBS containing bovine serum albumin for 90 min at room temperature. Detection of the biotin–streptavidin–peroxidase complex was carried out by incubating sections with DAB solution (1:1, 3,3’-diaminobenzidine in PBS and 0.05% H2O2). Sections incubated with reaction mixture without DNA polymerase were used as control.
Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL staining)
The TUNEL technique was performed using the ApopTag Peroxidase Kits (Intergen Co., Purchase, NY). First, 3% H2O2 in 0.05 M PBS was applied to sections for 5 min to quench endogenous peroxidases. After washing with PBS and equilibrating with the supplied solution, the sections were incubated with TdT enzyme at 37°C for 1 h. The reaction was stopped by washing with stop buffer for 10 min. Anti-digoxigenin peroxidase conjugate was then applied to the sections for 30 min at room temperature. DAB was used to visualize the TUNEL staining. The omission of the terminal deoxynucleotidyl transferase was used as the negative control.
Statistical Analysis
All data in this study are presented as mean ± SD. Data were analyzed with ANOVA with a Scheffé F test. Statistical significance was accepted at p < 0.05.
Results
Role of PAR-1 in reducing brain edema with thrombin preconditioning
In WT mice, intracerebral injection of 0.1 U thrombin (TPC) 72 hours before injection of 0.5 U thrombin significantly reduced brain edema in the ipsilateral anterior part of the cerebrum on day 1 after the 2nd injection compared to WT controls where saline was infused 72 hours before the injection of 0.5 U thrombin. However, in PAR-1 KO mice, TPC has no effect on brain water content (water content (%): 79.25 ± 0.51 in WT TPC group vs. 80.30 ± 0.18 in WT saline group and 79.96 ± 0.21 in KO TPC group, n = 6 per group, p < 0.01; Fig. 1A). Brain sodium ion content correlated with the brain water content such that reduced accumulation of sodium ion with TPC in WT mice was observed (sodium content: 213 ± 10 in WT TPC group vs. 242 ± 4 in WT saline group and 236 ± 9 mEq/kg dry wt in KO TPC group, n = 6 per group, p < 0.01; Fig. 1B).
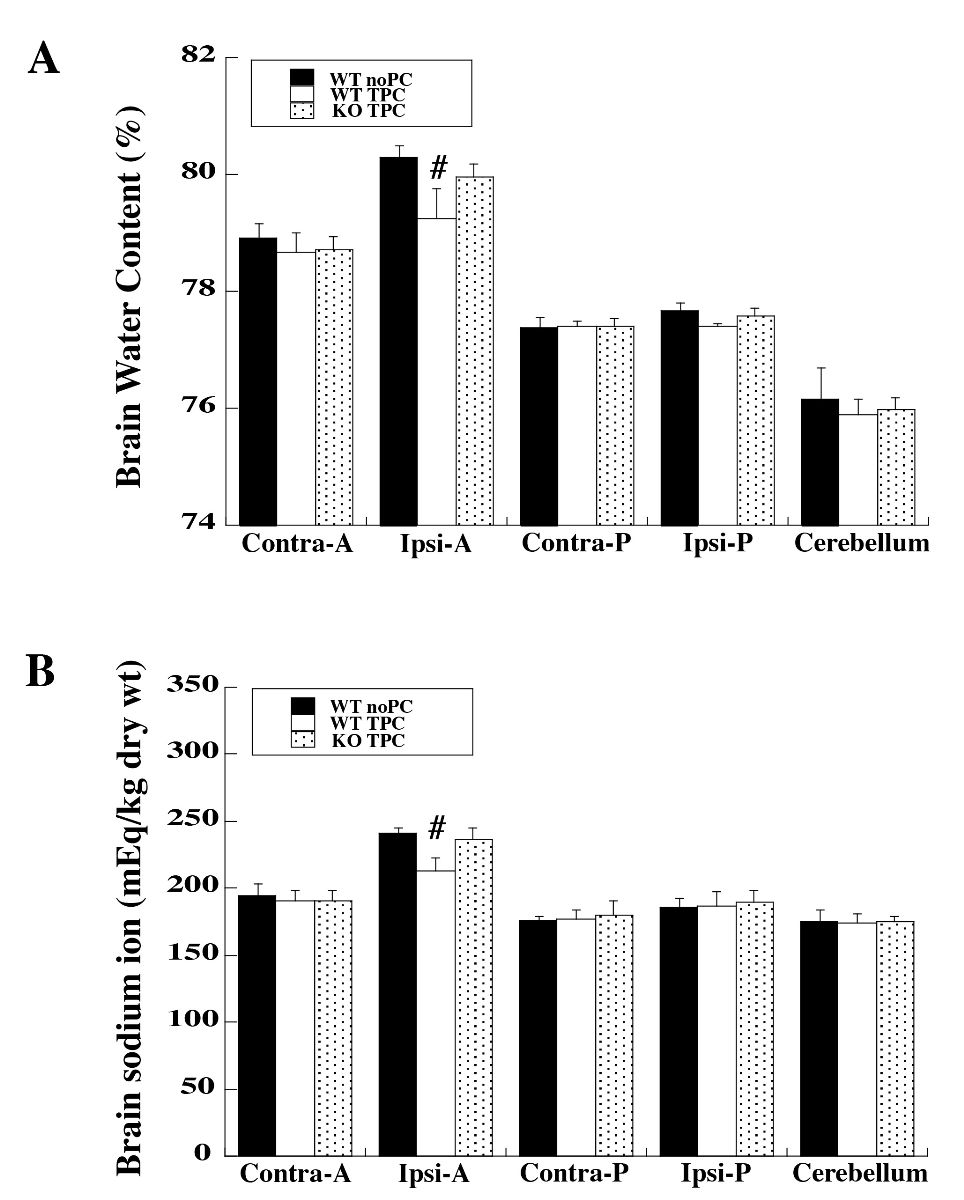
In a new window | Download PPT
Figure 1: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, (A) brain water and (B) sodium ion contents were determined. Five samples from each brain were analyzed: ipsilateral and contralateral anterior cerebrum (Ipsi-A and Contra-A), ipsilateral and contralateral posterior cerebrum (Ipsi-P and Contra-P), and cerebellum. Values are expressed as mean ± SD; n = 6 per group. #p < 0.01 vs. the other two groups.
Role of PAR-1 in reducing BBB disruption with thrombin preconditioning
Albumin leakage (Western blot) was used to evaluate the BBB disruption day 1 after the second injection. TPC 72 hours before injection of 0.5 U thrombin significantly decreased albumin leakage in the ipsilateral basal ganglia of WT mice compared with WT mice treated with saline, while no protective effect was found with TPC in the PAR-1 KO group (albumin (pixels): 2807 ± 607 in WT TPC group vs. 5312 ± 1818 in WT saline group and 5738 ± 1594 in KO TPC group, n = 4 per group, p < 0.05; Fig. 2A).
Role of PAR-1 in the beneficial effects of thrombin preconditioning on behavioral deficits
A corner turn test performed on day 1 after the second injection revealed that TPC 72 hours before injection of 0.5 U thrombin significantly decreased corner turn test scores in WT mice compared to WT saline controls. The corner turn test score in the PAR-1 KO TPC group was similar to the WT saline group (corner turn test scores (%): 47 ± 18 in WT TPC group, n = 7, vs. 85 ± 24 in WT saline group, n = 6, and 93 ± 11 in KO TPC group, n = 11, p < 0.01; Fig. 2B).

In a new window | Download PPT
Figure 2: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, brains were used to (A) assess albumin levels in the ipsilateral basal ganglia by Western blot analysis. Values are expressed as mean ± SD; n = 4 per group. *p < 0.05 vs. the other two groups. (B) Mice underwent behavioral testing (corner turn test) 24 hours after intracerebral injection of 0.5 U thrombin. Corner turn test scores are expressed as mean ± SD. WT noPC n = 6, WT TPC n = 7, KO TPC n = 11, #p < 0.01, vs. the other two groups.
Role of PAR-1 in thrombin preconditioning-induced protection against neuronal death and DNA damage
Neuronal degeneration and DNA strain breaks were measured 1 day after the second injection. There were markedly fewer Fluoro-Jade C positive cells in the basal ganglia of the WT TPC group than the WT saline group and the PAR-1 KO TPC group after 0.5 U-thrombin injection (Fluoro-Jade C positive cells/mm2: 312 ± 108 in WT TPC group vs. 760 ± 116 in WT saline group and 953 ± 182 in KO TPC group, n = 5 per group, p < 0.01; Fig. 3). Similarly, there were fewer cells positive for DNA damage in the WT TPC group compared to the other two groups (PANT positive cells/mm2: 239 ± 137 in WT TPC group vs. 781 ± 334 in WT saline group and 866 ± 272 in KO TPC group, n = 5 per group, p < 0.01; TUNEL positive cells/mm2: 318 ± 179 in WT TPC group, vs. 683 ± 224 in WT saline group and 630 ± 155 in KO TPC group, n = 5 per group, p < 0.05; Fig. 3).
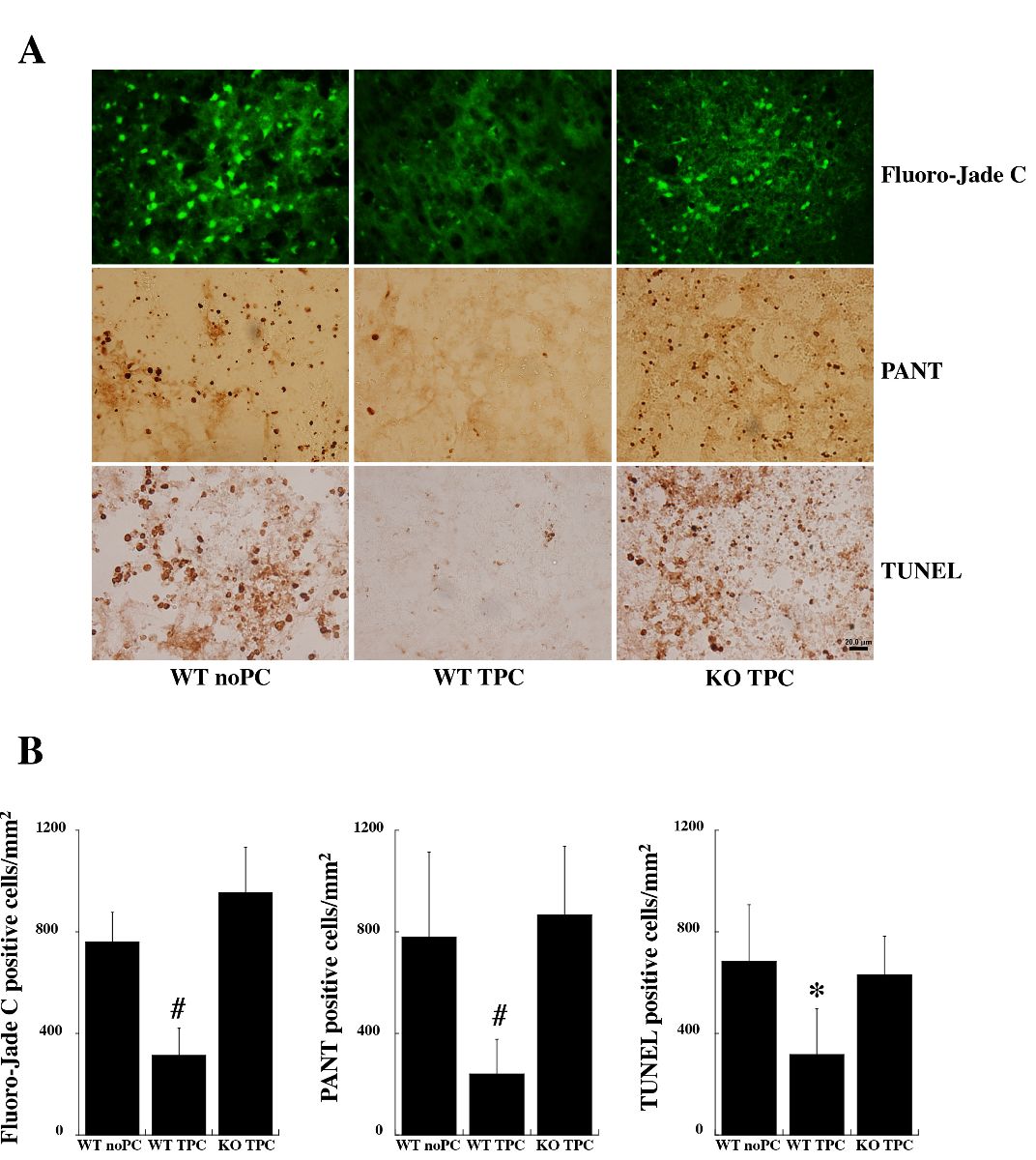
In a new window | Download PPT
Figure 3: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, brains were used to assess neuronal death and DNA breaks. (A) Fluoro-Jade C, PANT, TUNEL staining in the ipsilateral basal ganglia. (B) Number of positive cells expressed as mean ± SD. n = 5 per group, #p < 0.01, *p < 0.05 vs. the other two groups. Scale bar = 20 μm.
Role of PAR-1 in heat shock protein and ceruloplasmin upregulation after infusion with low dose of thrombin
Western blot analysis found that 72 hours after an intracerebral injection of 0.1 U thrombin the levels of HSP25 was significantly upregulated in the ipsilateral basal ganglia of WT mice compared with PAR-1 KO mice and WT saline mice (HSP25/β-actin ratio: 0.15 ± 0.08 in WT + 0.1 U thrombin vs. 0.02 ± 0.01 in WT + saline, and 0.03 ± 0.002 in KO + 0.1 U thrombin, n = 4 per group, p < 0.01; Fig. 4). A similar upregulation in the ipsilateral basal ganglia was found with HSP32 (HSP32/β-actin ratio: 0.15 ± 0.05 in WT + 0.1 U thrombin vs. 0.04 ± 0.02 in WT + saline, and 0.02 ± 0.01 in KO + 0.1 U thrombin, n = 4 per group, p < 0.01; Fig. 4)] and ceruloplasmin [ceruloplasmin/β-actin ratio: 0.22 ± 0.1 in WT + 0.1 U thrombin vs. 0.08 ± 0.01 in WT + saline, and 0.04 ± 0 .02 in KO + 0.1 U thrombin, n = 4 per group, p < 0.01; Fig. 5).

In a new window | Download PPT
Figure 4: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received an intracerebral injection of 0.1 U thrombin or saline. At 72 h, heat shock protein (HSP) 25 and HSP 32 levels in the ipsilateral basal ganglia were assessed by Western blot analysis. Values (ratio to β-actin) are expressed as mean ± SD, n = 4, #p < 0.01 vs. the other two groups.
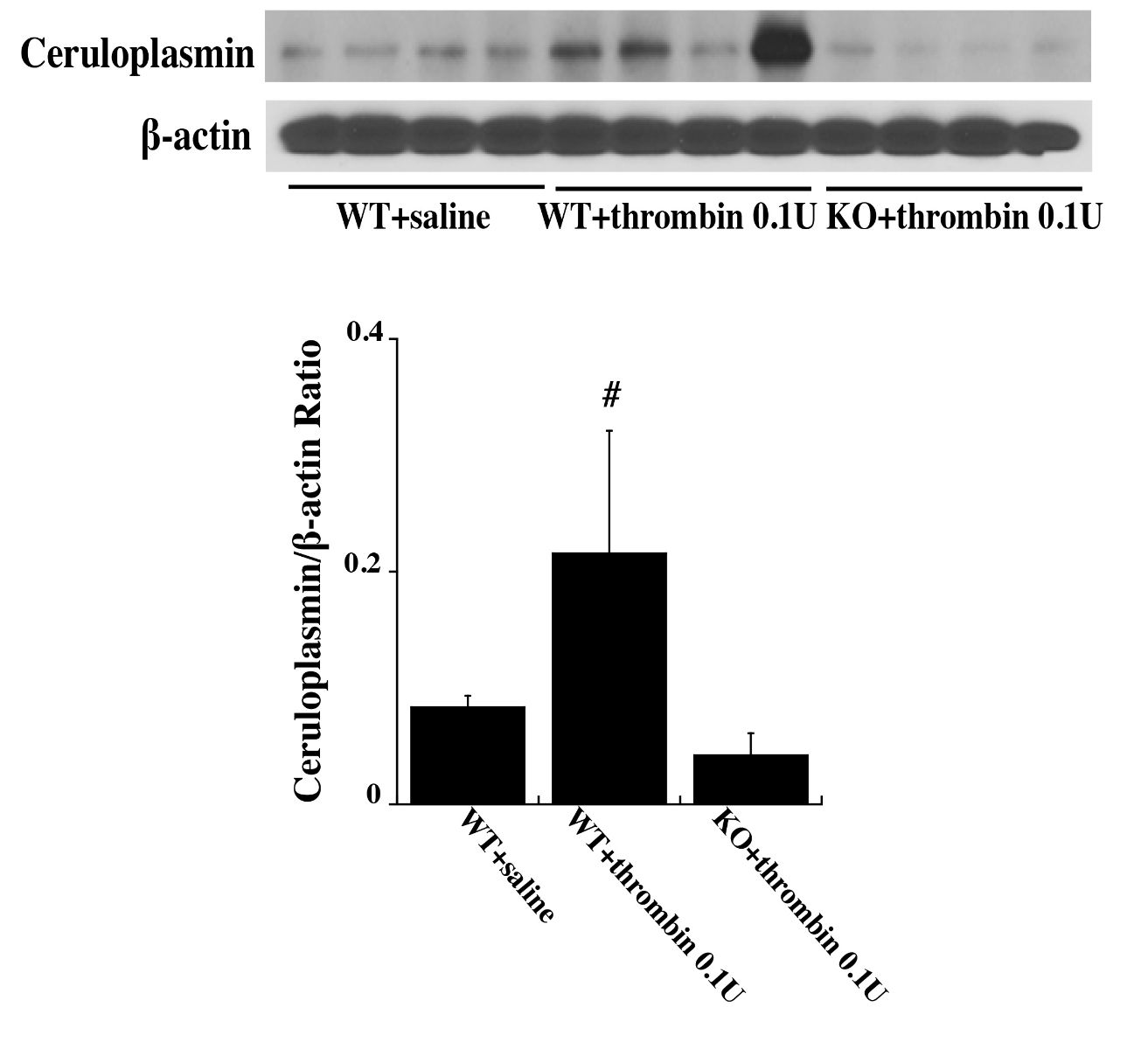
In a new window | Download PPT
Figure 5: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received an intracerebral injection of 0.1 U thrombin or saline. At 72 h, ceruloplasmin levels in the ipsilateral basal ganglia were assessed by Western blot analysis. Values (ratio to β-actin) are expressed as mean ± SD, n = 4, #p < 0.01 vs. the other two groups.
Discussion
This study reports the following findings: 1) preconditioning with low dose thrombin resulted in less brain injury following a subsequent injection of a high dose of thrombin, including reduced brain swelling, BBB disruption, neuronal death, DNA damage, and neurological function deficits. 2) These protective effects of TPC were absent in PAR-1 KO mice. 3) Low dose thrombin upregulated heat shock proteins (HSP25 and HSP32) and ceruloplasmin in WT but not in PAR-1 KO mice. These results suggest that PAR-1 may play an important role in thrombin preconditioning against thrombin-induced brain injury and that this is associated with upregulation of a series of potentially neuroprotective proteins.
Previous studies indicate that preconditioning stimuli induce a variety of endogenous protective mechanisms in the brain (Gidday, 2006; Yokobori et al., 2013; Stetler et al., 2014; Zhao et al., 2017). Thrombin is a serine protease produced immediately in the brain after cerebral hemorrhage or with BBB disruption that follows many kinds of brain injury. Direct injection of large doses of thrombin into the brain causes severe injury including inflammatory cell infiltration, brain edema formation, and an increase in reactive astrocytes (Xi et al., 1999; Krenzlin et al., 2016). However, our prior studies demonstrated that pretreatment with low doses of thrombin can reduce the brain injury caused by hemorrhage, ischemia, iron, or large doses of thrombin, both in vivo and in vitro (Jiang et al., 2002; Hua et al., 2003; Yang et al., 2006; Bao et al., 2018). Nevertheless, the precise mechanisms of thrombin-induced protection have yet to be elucidated. This study used PAR-1 KO mice to explore the role of that receptor in TPC in vivo.
The results in this study indicate that thrombin-induced neuroprotective effects might be initiated through PAR-1, consistent with previous studies (Jiang et al., 2002; Bao et al., 2018). PAR-1 was the first discovered member of a unique class of G-protein-coupled signaling receptors that become activated via cleavage of their extracellular amino terminus by proteases such as thrombin (Flynn and Buret, 2004; Zhen et al., 2016). Striggow et al. (2000) have shown that the protective effect of thrombin is caused only by concentrations in the picomolar range and is mediated by PAR-1 activation. They also found that PAR-1 is upregulated in the hippocampus by severe ischemia in organotypic hippocampal slice cultures (Striggow et al., 2001). The present study revealed that TPC had a wide range of neuroprotective effects against later brain injury induced by a large dose of thrombin, including reducing brain edema formation and BBB disruption, attenuating neuronal death and DNA injury, and preserving normal behavioral function. More importantly, all of the TPC neuroprotective effects were absent in PAR-1 KO mice. This suggests PAR-1 plays an essential role in the neuroprotective effects of thrombin preconditioning.
In addition, this study found that a low dose of thrombin induced upregulation of HSP25 and HSP32 in the brains of WT but not PAR-1 KO mice. HSP25/27 (depending on species) is reported to perform multiple cellular protective functions including mediating cell survival directly or undergoing recognition and chaperoning of damaged or misfolded proteins (Stetler et al., 2009; Shi et al., 2017). Stetler et al. (2008) reported that HSP27 protects against ischemic brain injury by attenuating a novel stress-response cascade upstream of mitochondrial cell death signaling. van der Weerd et al. (2010) found that overexpression of HSP27 in transgenic mice reduces cortical damage after cerebral ischemia. HSP32, also called hemeoxygenase-1 (HO-1), is a stress protein and the rate-limiting enzyme in the heme degradative pathway, which may play an important role in cytoprotection against oxidative injury as well as heme- and hemoglobin-induced toxicity (Xi et al., 1999; Sharp et al., 2013). Intracerebral injection of a low dose of thrombin was found to induce an increase in HSP32 immunoreactivity in our previous rat models (Xi et al., 1999), suggesting HSP32 could also be involved in TPC. Our findings indicate that TPC induced upregulation of HSP 25 and HSP 32 is via PAR-1 activation.
Furthermore, a low dose of thrombin upregulated ceruloplasmin in the brain of WT but not PAR-1 KO mice. Ceruloplasmin is involved in iron metabolism by oxidizing ferrous iron to ferric iron (Liu et al., 2019). Our previous studies have shown that pretreatment with low-dose thrombin attenuates brain edema induced by iron (Hua et al., 2003), and TPC increases brain ceruloplasmin mRNA levels and exogenous ceruloplasmin reduces ferrous iron-induced brain edema (Yang et al., 2006). Chen-Roetling et al. (2012) reported that iron chelators could antagonize the beneficial effect of TPC. In light of these data and our present results, we hypothesize that TPC may attenuate iron associated injury by upregulating ceruloplasmin via PAR-1.
The current study demonstrates the importance of PAR-1 in TPC-induced protection in vivo. However, the importance of understanding the role of PAR-1 activation in brain protection is not limited to thrombin and TPC. PAR-1 is also activated by activated protein C (APC), although by cleavage at a different site (Arg46) than thrombin (Arg41) (Griffin et al., 2018). APC has anti-apoptotic, anti-inflammatory, and neuro- and vasculo-protective effects where PAR-1 plays a critical role (Griffin et al., 2018). A mutant form of APC, 3K3A-APC, which has reduced anti-coagulant activity, is being examined clinically as an adjunct therapy for tissue plasminogen and thrombectomy in ischemic stroke (Lyden et al., 2019). The potential of 3K3A-APC as preconditioning agent merits investigation.
There are several limitations in the current study. For example, only female mice were used. Whether or not estrous cycle-related hormonal changes affect preconditioning was not tested. However, it should be noted that thrombin-induced brain protection has been tested in males (Xi et al., 1999). In addition, a PAR-1 knockout group without preconditioning was not included in this study.
In conclusion, our data demonstrate that PAR-1 plays an important role in thrombin preconditioning against thrombin-induced brain injury in female mice. This may be linked to the role of PAR-1 in upregulating a series potentially neuroprotective proteins including heat shock proteins (HSP25, 32) and the iron-handling protein (ceruloplasmin). Our findings could lead to a better understanding of the mechanisms of thrombin preconditioning as well as its potential application in clinical settings.
References
Xuhui Bao1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Fenghui Ye1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Shu Wan1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Ya Hua1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Richard F. Keep1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Hugh J. L. Garton1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Guohua Xi1
1Department of Neurosurgery, University of Michigan, Ann Arbor, MI, USA.
Corresponding author:
Guohua Xi
Email: guohuaxi@umich.edu

In a new window | Download PPT
Figure 1: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, (A) brain water and (B) sodium ion contents were determined. Five samples from each brain were analyzed: ipsilateral and contralateral anterior cerebrum (Ipsi-A and Contra-A), ipsilateral and contralateral posterior cerebrum (Ipsi-P and Contra-P), and cerebellum. Values are expressed as mean ± SD; n = 6 per group. #p < 0.01 vs. the other two groups.

In a new window | Download PPT
Figure 2: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, brains were used to (A) assess albumin levels in the ipsilateral basal ganglia by Western blot analysis. Values are expressed as mean ± SD; n = 4 per group. *p < 0.05 vs. the other two groups. (B) Mice underwent behavioral testing (corner turn test) 24 hours after intracerebral injection of 0.5 U thrombin. Corner turn test scores are expressed as mean ± SD. WT noPC n = 6, WT TPC n = 7, KO TPC n = 11, #p < 0.01, vs. the other two groups.

In a new window | Download PPT
Figure 3: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received intracerebral injections of 0.1 U thrombin (thrombin preconditioning, TPC) or saline (no PC) followed by a large dose of thrombin (0.5 U) at 72 hours. Twenty-four hours after intracerebral injection of 0.5 U thrombin, brains were used to assess neuronal death and DNA breaks. (A) Fluoro-Jade C, PANT, TUNEL staining in the ipsilateral basal ganglia. (B) Number of positive cells expressed as mean ± SD. n = 5 per group, #p < 0.01, *p < 0.05 vs. the other two groups. Scale bar = 20 μm.

In a new window | Download PPT
Figure 4: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received an intracerebral injection of 0.1 U thrombin or saline. At 72 h, heat shock protein (HSP) 25 and HSP 32 levels in the ipsilateral basal ganglia were assessed by Western blot analysis. Values (ratio to β-actin) are expressed as mean ± SD, n = 4, #p < 0.01 vs. the other two groups.

In a new window | Download PPT
Figure 5: Wild type (WT) and protease activated receptor-1 knockout (KO) mice received an intracerebral injection of 0.1 U thrombin or saline. At 72 h, ceruloplasmin levels in the ipsilateral basal ganglia were assessed by Western blot analysis. Values (ratio to β-actin) are expressed as mean ± SD, n = 4, #p < 0.01 vs. the other two groups.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7780 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA