International bi-monthly journal of cell signaling, tissue protection, and translational research.
Epigenetic Regulation by Dietary Restriction: Part II
Gavin Yong-Quan Ng1, David Yang-Wei Fann1, Dong-Gyu Jo2, Christopher G. Sobey3, Thiruma V. Arumugam1,2,3
Author Affiliations


- 1Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 2School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea.
- 3Department of Physiology, Anatomy & Microbiology, School of Life Sciences, La Trobe University, Bundoora, Victoria, Australia.
Abstract
In the first part of our review, we extensively discuss the different variants of dietary restriction (DR) regimens, as well as its corresponding mechanism(s) and subsequent effects. We also provide a detailed analysis of the different epigenetic mechanisms based on current knowledge. We postulate that DR may represent an environmental intervention that can modulate the epigenomic profile of an individual. It is highly plausible that epigenetic regulation by DR may help explain the asymmetric manifestation of DR effects in different individuals. Additionally, epigenetic modifications via DR may lead to epigenetic programming, providing protection against age-associated diseases, which in turn could lead to reduced morbidity and increased lifespan. In the second part of the review, we summarize recent findings that highlight the epigenomic axis of DR, which provides a better understanding of the mechanisms by which its numerous health benefits are achieved.
Keywords: dietary restriction, epigenetics, aging, caloric restriction, intermittent fasting, fasting-mimicking diet, DNA methylation, histone modifications, histone remodelling, microRNAs
Abstract
In the first part of our review, we extensively discuss the different variants of dietary restriction (DR) regimens, as well as its corresponding mechanism(s) and subsequent effects. We also provide a detailed analysis of the different epigenetic mechanisms based on current knowledge. We postulate that DR may represent an environmental intervention that can modulate the epigenomic profile of an individual. It is highly plausible that epigenetic regulation by DR may help explain the asymmetric manifestation of DR effects in different individuals. Additionally, epigenetic modifications via DR may lead to epigenetic programming, providing protection against age-associated diseases, which in turn could lead to reduced morbidity and increased lifespan. In the second part of the review, we summarize recent findings that highlight the epigenomic axis of DR, which provides a better understanding of the mechanisms by which its numerous health benefits are achieved.
Keywords: dietary restriction, epigenetics, aging, caloric restriction, intermittent fasting, fasting-mimicking diet, DNA methylation, histone modifications, histone remodelling, microRNAs
1.0 Introduction
Following sexual maturation, aging is characterized by a progressive decline in biological processes through a loss in molecular fidelity, and is a major risk factor for development of many chronic diseases. While the underlying mechanisms of aging involve many specific hallmarks and conserved biological pathways, we still have a poor understanding of the links between aging and development of age-associated diseases due to the complexities of genetic, environmental, and stochastic factors. As a result, strategies to significantly promote extension of health span during aging have been unsuccessful. Recent studies have shown that aging possess an epigenetic component, with many age-associated epigenetic changes underlying hallmarks of aging. For example, aging has been implicated in alterations to DNA methylation, to imbalance in histone modifications, to chromatin remodeling, and with an involvement of many miRNAs. As such, it follows that aging may also be driven by extensive epigenomic remodeling, which may in turn promote the development of age-associated diseases. Because epigenetic modifications may be modifiable by environmental changes and exhibit immense plasticity, they could be used transduce external signals and regulate aging through gene regulation. It has been widely reported that DR can promote changes in gene expression and attenuate age-associated changes across many organisms (Lee et al., 1999; Swindell, 2009; Plank et al., 2012; Choi et al., 2013; Whitaker et al., 2014; Wood et al., 2015; Hadad et al., 2016; Cheng et al., 2017; Wei et al., 2017; Kim et al., 2018; Malinowski et al., 2019; Ng et al., 2019). DR, as previously defined in the first part of our review, is a voluntary abstinence from consumption of a selected or entire nutrient composition without compromising energy balance or inducing malnutrition (Masoro, 1998; Robertson and Mitchell, 2013). Variants of DR regimens have emerged over the years, and can be broadly classified as either caloric restriction (CR) or intermittent fasting (IF) (Lee and Longo, 2016), as well as the new ‘fasting mimicking diet’ (FMD) (Wei et al., 2018). Since DR impacts both lifespan and health span in a plethora of model organisms, it is plausible that nutrient availability can also drive epigenetic remodeling to impact aging. In this second part of the review, we will describe the relationship between DR and the epigenome (Figure 1 and Table 1).
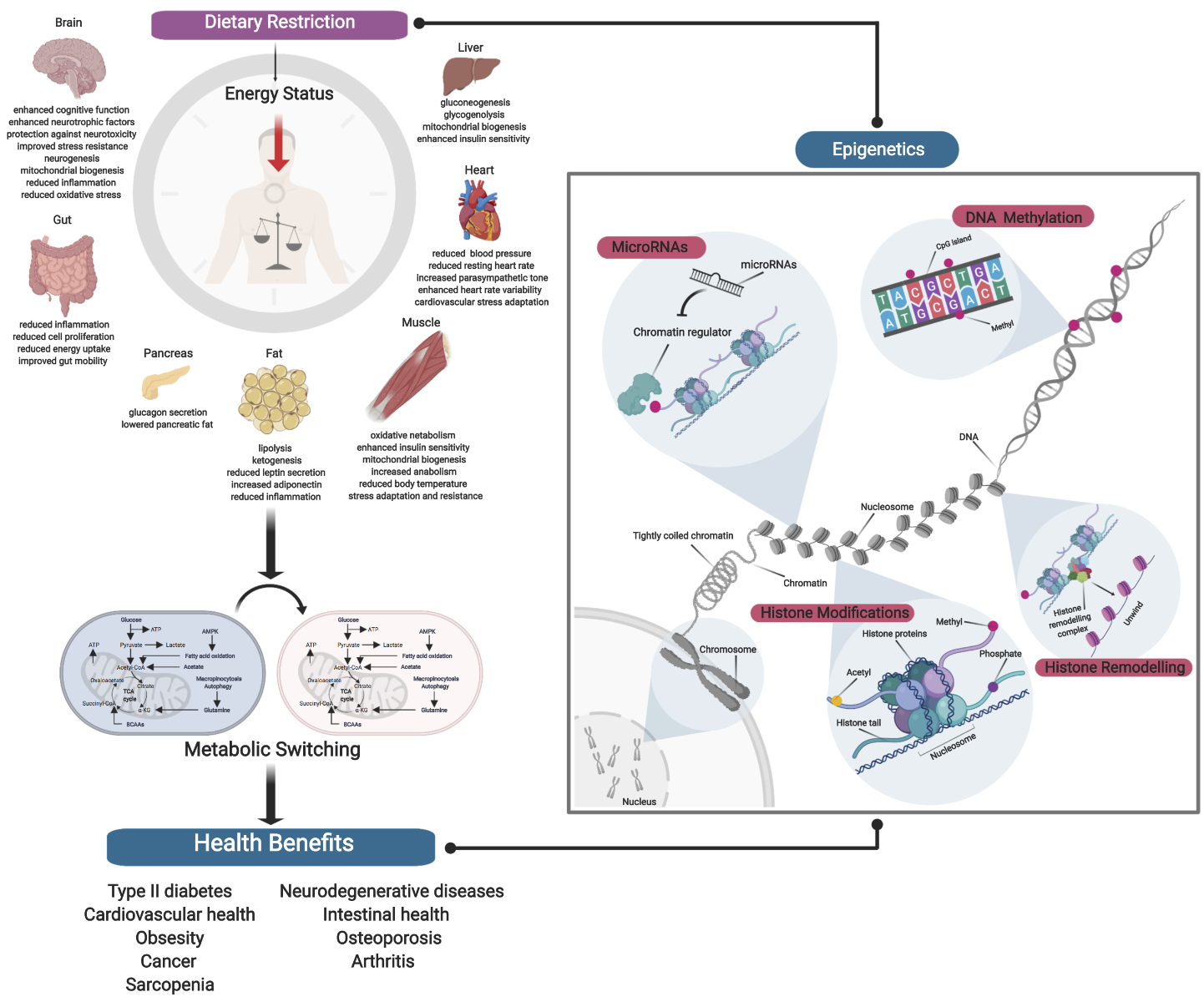
In a new window | Download PPT
Figure 1: Dietary Restriction and Epigenetics. Schematic diagram showing robust systemic effects exerted by DR, as well as an overview of the different epigenetic mechanisms. Establishing the relationship between DR and epigenetics is critical in deciphering the epigenetic milieu of DR in producing differential responses. This may help to explain the asymmetric manifestation of DR effects, as well as allowing us to better understand the epigenetic regulation of DR by which its numerous health benefits are achieved.
Table 1. Different Types and Effects of Dietary Restriction Regimens.
2.0 Dietary Restriction and DNA Methylation
DR has been widely reported to influence age-associated genes expression via DNA methylation across a wide range of models and species. For example, in the relatively simple invertebrate model of Daphnia magna, DNA methylation changes following CR were observed to control expression of genes related to methylation and acyl-Coenzyme A dehydrogenase (Hearn et al., 2019). Such a phenomenon can also be demonstrated in rodents. CR can protect against age-associated methylation in the liver of mice (Miyamura et al., 1993; Cole et al., 2017). CR initially represses DNA methylation in mice, but interestingly the effects may become less prominent with age. Notably, in the liver of mice, CR can suppress age-dependent increases in c-myc gene methylation (Miyamura et al., 1993) and suppress changes in DNA methylation (Hahn et al., 2017), which drives epigenetic reprogramming of lipid metabolic processes, leading to a metabolic switch towards reduction in triglyceride levels and short-chain triglyceride-associated fatty acids that is more pronounced with age (Hahn et al., 2017). On the other hand, CR increased ras DNA methylation in rat pancreatic acinar cells such that across generations, CR cells demonstrated reduced oncogene expression and mutations, including reduced p53 tumor suppressor gene mutation, proliferation, and transformation (Hass et al., 1993). In rat kidney, CR induced increased DNA methylation in promoter and intronic regions, which repressed pathways associated with age-associated diseases such as cancer and diabetes (Kim et al., 2016). In the aged mouse brain, CR induced attenuation of DNA methylation at both the cytosine-guanine (CG) and non-CG (CH) sites in the hippocampus, leading to activation of neuroprotective pathways, and impacted cognitive function via DNA methyltransferase 3a (DNMT3a) (Chouliaras et al., 2011). CR can also suppress age-associated increases in DNA methylation and hydroxymethylation in cerebellar Purkinje cells, pointing to a spatial regulation of DNA methylation in different brain regions (Lardenoije et al., 2015). In a mouse model of female breast cancer, CR induced hypermethylation at CpG sites for transcriptional regulator CCCTC-binding factor of both estrogen receptor 1 and 2 (ESR1 and ESR2), leading to transcriptional activation and gene expression of both estrogen receptor alpha and beta (ERα and ERβ). CR also attenuated the obesity-associated increase in DNA methyltransferase 1 (DNMT1) DNA methylation, and reduced development of mammary tumorigenesis. Therefore, an intervention with CR may exert a crucial role in modulating DNA methylation to prevent Herceptin 2 (HER2)-positive breast cancer development (Rossi et al., 2017).
While DNA methylation is tightly coordinated, collective gains and losses of DNA methylation, termed ‘epigenetic drift’, has been described in mice, rhesus monkeys, and humans during aging (Maegawa et al., 2017). Blood studies in mice showed that unique DNA methylation signatures became more prominent with age and contributed towards aging following remodeling of the genome. CR has been shown to remodel these DNA methylation signatures to contribute to longevity (Sziráki et al., 2018). Moreover, a study on blood from rhesus monkeys showed CR to attenuate this age-associated DNA methylation drift, an effect that correlates with increased lifespan (Maegawa et al., 2017).
Effects of DR on DNA methylation have also been widely studied in humans. In Caco-2 human epithelial colorectal adenocarcinoma cells and human umbilical vein endothelial cells, Sirt1 mediates DNA methylation by DR to control differential gene expression (Ions et al., 2013). Glucose restriction in WI-38 cells and immortalized WI-38/S cancer cells induces changes in DNA methylation and subsequent chromatin remodeling at both hTERT and p16 promoter regions. Notably, normal WI-38 cells exhibit increased longevity after glucose restriction, whereas WI-38/S cancer cells exhibit growth inhibition and apoptosis, providing an insight into how DR and DNA methylation might be used to improve cancer treatment (Li et al., 2019). Interestingly, postmenopausal women who were either overweight or obese possessed significant DNA methylation differences at 35 loci prior to CR in their subcutaneous adipose tissue, but showed a reduction to only three significant DNA methylation differences following it. At these three loci, DNA methylation controls genes involved in body weight control, insulin secretion, and genome imprinting (Mill et al., 2010). Moreover, in another study performed in obese women using blood samples or peripheral blood mononuclear cells, similar methylation patterns were observed in hypomethylation of both leptin and tumor necrosis factor-alpha (TNF-α) promoter regions following CR-induced weight loss (Cordero et al., 2011). Other genes potentially controlled by DNA methylation modulation include cluster determinant 36 (CD36), cluster determinant 14 (CD14), pyruvate dehydrogenase kinase 4 (PDK4), and fatty acid desaturase 1 (FADS1) (Amaral et al., 2014). Furthermore, TNF-α is a proinflammatory cytokine and obese men subjected to CR-induced weight loss demonstrated hypomethylation at the TNF-α promoter region in blood mononuclear cells (Campión et al., 2009). A similar study in peripheral blood mononuclear cells from overweight or obese men found key changes in DNA methylation loci at the ATPase phospholipid transporting 10A (ATP10A), cluster determinant 44 (CD44), and Wilms tumor 1 (WT1) genes induced by CR (Milagro et al., 2011). In addition to CR, overnight and 36-hour fasting in young low birthweight and normal weight subjects demonstrated hypermethylation in both leptin (LEP) and adiponectin (ADIPOQ) genes from plasma samples, which positively correlated with total body fat composition (Hjort et al., 2017). Concordantly, it appears that DNA methylation changes at key gene loci can be used as an early indicator of response to human weight-loss interventions.
Interestingly, in patients with morbid obesity, for which bariatric surgery is used as an option to induce weight loss, their reduced food intake due to a smaller stomach capacity results in a mode of CR. In one study, obese nondiabetic patients placed on a very low caloric diet only exhibited hypomethylation of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A) in their blood, whereas those who underwent a Roux-en Y gastric bypass (RYGB), showed altered levels of methylation in PPARGC1A, transcription factor A (TFAM), interleukin-1 beta (IL1-β), interleukin-6 (IL-6), and in TNF-α promoter regions with corresponding hypermethylation in PDK4, IL1-β, IL-6, and TNF-α after one year. It has also been reported that levels of PPARGC1A, PDK4, sorbin, and SH3 domain containing 3 (SORBS3) DNA methylation were altered in skeletal muscle following RYGB (Barres et al., 2013; Day et al., 2017). Furthermore, in adipose tissue, DNA methylation of many CpG sites of promoter regions, such as cholesteryl ester transfer protein (CETP), forkhead box P2 (FOXP2), HDAC4, DNMT3B, potassium voltage-gated channel subfamily Q member 1 (KCNQ1), and Hox, are altered following gastric bypass (Benton et al., 2015). Thus, it appears that CR-relevant surgical interventions to treat obesity such as RYGB may have a robust impact on DNA methylation of a myriad of genes, providing an impetus for further investigation of the associations of DNA methylation with obesity, and the mechanisms underlying improvements in metabolic health following surgery.
Notably, DR-induced changes in DNA methylation can result in epigenetic reprogramming and maintenance that may also subsequently affect future generations of offspring. For example, DR during maternal pregnancy in mice resulted in intrauterine growth restriction (IUGR) of the fetus, which promoted the development of chronic diseases (such as glucose intolerance, increased fat deposition, as well as hypercholesterolemia) in adulthood of the male offspring. It appears that DR can influence the placental environment via genome-wide hypomethylation (Chen et al., 2013). Interestingly, people impacted by the Dutch famine exhibited DNA methylation changes in whole blood during early gestation, but not in mid or late gestation (Tobi et al., 2015). By contrast, in pregnant rats CR was found to alter liver expression of the fatty acid synthase (Fasn) gene and blood cholesterol levels, without detectable changes in DNA methylation such that no effects were transferred to the offspring (Nowacka-woszuk et al., 2017; 2018, 2019). Apparent variance in transgenerational effects of DR between studies may be due to organism- or organ-specific effects, and/or lack of study of genome-wide DNA methylation. In considering the implications of epigenetic modifications, it is therefore important to consider that wider gene expression changes may be modulated as a result of DNA methylation changes.
3.0 Dietary Restriction and Histone Modification/Remodeling
Histone deacetylation catalyzed by histone deacetylases (HDACs) is a widely studied histone modification occurring during DR. The four identified classes within this family are: class I HDACs (HDAC1, HDAC2, HDAC3, and HDAC8); class II HDACs (HDAC4, HDAC5, HDAC6, HDAC7, HDAC9, and HDAC10); class III HDACs (SIRT1, SIRT2, SIRT3, SIRT4, SIRT5, SIRT6, and SIRT7); and class IV HDACs (HDAC11). Class I HDACs are homologous to yeast Rpd3 HDAC complex, and class IV HDACs are most closely related to class I HDACs. On the other hand, class II HDACs possess homologous domains to a yeast enzyme Hda1, and class III HDACs are homologous to yeast silent mating type information regulation 2 (Sir2).
As for the established relationship between glucose restriction and DNA methylation in normal and immortalized WI-38 and WI-38/S cells respectively, the roles of HDAC1 have been similarly investigated (Li et al., 2019). Glucose restriction causes a decrease in HDAC1 activity around the transcriptional initiation site of human telomerase reverse transcriptase (hTERT) in both normal WI-38 and immortalized WI-38/S cells, whereas an increase in HDAC1 activity occurs around the transcriptional initiation site of p16 promoter region in normal WI-38 cells and there is a loss of HDAC1 binding at the p16 promoter in immortalized WI-38/S cells. As a result, an alteration in gene expression level of both hTERT and p16 genes have differential beneficial effects on fates of both normal and precancerous cells, favoring longevity in normal cells and apoptosis in precancerous cells (Li et al., 2019). In another notable observation, an increase in Hdac1 gene was found in livers of the first two generations of offspring from rats subjected to CR during pregnancy, but a decrease was observed in the third generation. Moreover, a global histone H3 acetylation was also observed in the fetal liver in both the first and second generation. Interestingly, while rats do not demonstrate transgenerational inheritance of DNA methylation changes following CR during pregnancy, it appears that prenatal CR can indeed modulate histone modifications with transgenerational effects (Nowacka-Woszuk et al., 2018).
Aging increases the level of HDAC2 in mouse hippocampus, with CR able to attenuate this increase. As the level of HDAC2 correlates with 5-methylcytidine DNA methylation in the nucleus of hippocampal cells, it demonstrates that CR can prevent age-associated changes in both histone modifications and DNA methylation (Chouliaras et al., 2013). As for HDAC3, HDAC3 knockout mice exhibit increased bone marrow fat, a tissue that produces adiponectin during CR, to control metabolic activity of nearby muscle. Thus, HDAC3 may play a key role in modulating metabolism of skeletal muscle during CR (McGee-Lawrence et al., 2016). Furthermore, fasted mice exhibit increased levels of HDAC3 and HDAC4 in the medial hypothalamus, and exhibit fewer acetylated histones H3 and H4 cells in the ventrolateral subdivision of the ventromedial hypothalamus. Thus, HDAC3 and HDAC4 are implicated in the modulation of hypothalamic gene expression in response to fasting (Funato et al., 2011). Overnight fasting in mice can promote cyclic adenine monophosphate signaling to increase binding of HDAC4 and HDAC5 to glucose transporter protein 4 (GLUT4) promoter region, thus decreasing expression of GLUT4 mRNA. As such, HDAC4 and HDAC5 may play important physiological roles in modulating metabolic homeostasis in adipose tissue in response to fasting (Weems et al., 2012). Furthermore, HDAC4 has been shown to be modulated during fasting conditions in Drosophila. During short-term fasting, activation of adipokinetic hormone (AKH) pathway will modulate lipid storage by inhibiting liver kinase B1 (LKB1) and inducing HDAC4 nuclear localization to alter brummer gene expression. However, during prolonged fasting, an AKH-independent signaling pathway will downregulate LKB1-salt inducible kinase 3 (SIK3) pathway to induce lipolysis. As such, the involvement of LKB1-SIK3-HDAC4 axis in Drosophila to modulate lipid homeostasis during fasting is highly dependent on the fasting duration as well as the regulatory partners (Choi et al., 2015). A similar study of Drosophila during fasting has shown that FOXO activity is regulated via this axis (Crunkhorn, 2011). This pathway also appears to exist in mice, where inhibition of SIK2 (mouse SIK3 homologue) induces HDAC4 dephosphorylation to promote gluconeogenic gene transcription following glucagon injection to mimic fasting (Crunkhorn, 2011; Wang et al., 2011). Consistent with these findings, rapid dephosphorylation of class IIa HDACs (HDAC4 and HDAC5) occurring after glucagon injection drives their translocation to the nucleus and promotes binding to the gluconeogenic enzymes glucose-6-phosphatase (G6Pase) promoter region in the liver of mice. Moreover, once in the nucleus, both HDAC4 and HDAC5 recruit a class I HDAC member, HDAC3, to induce the deacetylation and activation of FOXO family of transcription factors and driving the induction of transcription of gluconeogenesis gene (Mihaylova et al., 2011).
Class III HDACs, or SIRTs, have received special attention due to their strong impact on aging and longevity. There are seven mammalian isoforms within the SIRT family, categorized according to their localization and cognate function. SIRT1, SIRT2, and SIRT6 are localized in the nucleus and act upon histone proteins or a myriad of proteins that influence transcription. SIRT3, SIRT4, and SIRT5 participate in redox reactions and metabolic processes in mitochondria. SIRT7 participate in cell cycle division within the nucleolus (Guarente, 2007; Sack and Finkel, 2012). Notably, SIRT1 and SIRT2 have been reported to be localized in the cytosol (Kupis et al., 2016).
SIRTs are NAD+-dependent deacetylases which act upon a plethora of protein targets and largely on histone terminal tails at lysine residues (Mitchell et al., 2018), and may. perform ADP-ribosylation for gene silencing and protein acylation within the mitochondria (Tanny et al., 1999; Carrico et al., 2018). As such, activities of SIRTs extend beyond their function as histone deacetylases. The first identified SIRT stems from a yeast ortholog, Sir2, which is linked to promotion of longevity. Genetic ablation of Sir2 reduces, whereas overexpression extends lifespan (Bordone et al., 2007). Subsequent studies established the relationship between dSir2 in Drosophila, Sir-2.1 in Caenorhabditis elegans (C. elegans), and the mammalian ortholog SIRT1 in mice with lifespan extension (Tissenbaum and Guarente, 2001; Rogina and Helfand, 2004; Satoh et al., 2013; Whitaker et al., 2013), highlighting the conserved importance of sirtuin in mediating longevity.
CR induces the activity of sirtuin, such as SIRT1, SIRT2, SIRT3, and SIRT6, and represses the activity of SIRT4 in mammals (Cohen et al., 2004; Shi et al., 2005; Wang et al., 2007; Chen et al., 2008; Kanfi et al., 2008). DR has also been shown to increase the expression of sirtuins. For instance, during IF, SIRT3 expression is increased which in turn leads to an increase in neuronal resistance against excitotoxicity (Liu et al., 2019). Consequently, sirtuin actions are functionally linked to the chromatin, where it exerts its influence over a myriad of histone modifications. For example, SIRT1 plays a critical role in maintaining the structure of two modes of heterochromatin, namely facultative and constitutive. Facultative heterochromatin refers to regions of chromatin that become tightly packed in response to biological processes like development, and can be restored to euchromatic structure through a reversible process. Constitutive heterochromatin refers to regions of chromatin permanently tightly packed once established, which are often irreversible, and which are often found at pericentromeric or telomeric regions to maintain integrity of chromosomal structure (Trojer and Reinberg, 2007). For example, SIRT1 mediates the histone deacetylation of histone 4 lysine 16 acetylation (H4K16Ac) and histone 3 lysine 9 acetylation (H3K9Ac) to promote facultative chromatin structure, and it can deacetylate linker histone 1 lysine 26 acetylation (H1bK26Ac) to form higher order chromosomal structure. SIRT1 activity can also influence transcriptional status, such as via mediating the loss of histone 3 lysine 79 dimethylation (H3K79me2), a histone mark that associates with transcriptional activation (Vaquero et al., 2004). Interestingly, some sirtuin family members may exert their actions at overlapping targets, such as SIRT2 and SIRT6, which can deacetylate histone 3 lysine 9 acetylation (H3K9Ac) loci implicated in mitosis and telomeric maintenance respectively (Vaquero et al., 2006; Michishita et al., 2008).
Many studies conclude that there is a causal link between CR and sirtuin-mediated downstream effects. While induction of sirtuin can promote lifespan extension, this phenomenon may either be functionally linked to CR or independent of CR. In the case of Drosophila, CR can activate dSir2 via HDAC Rpd3 to promote lifespan extension (Rogina and Helfand, 2004). On the other hand, Sir-2.1 is activated via the insulin-like signaling pathway to promote lifespan in C. elegans in a CR-independent manner (Tissenbaum and Guarente, 2001). As a result, it is paramount to consider the upstream signaling pattern of sirtuin to better understand the mechanistic relationship between CR and sirtuin. A controversial study has reported that SIRT activities may be tissue-specific. For instance, while CR can upregulate SIRT1 activity, downregulation is observed in the liver of mice (Chen et al., 2008). The notion that the effects of sirtuin vary across organs and organisms is evidence of their overall complexity. In-depth reviews of this topic have been published previously (Vaquero and Reinberg, 2009; Li et al., 2011).
Besides histone deacetylation, other types of histone modifications can occur as a result of DR. Specifically, CR reduces the level of N-terminal histone acetylation at histone H4 (NacH4), which upregulates stress-response genes such as pyrazinamidase/nicotinamidase 1 (Pnc1), which then regulates Sir2 activity and is important for longevity effects (Molina-Serrano et al., 2016). Histone acetylation is also greatly enhanced at the promoter region of mitochondrial activating genes in the skeletal muscle of mice fasted for 72 hours. Correspondingly, glucose tolerance, body weight, and exercise endurance are improved (Miyashita et al., 2019). Furthermore, a study of in utero undernutrition (50% CR) in rats during the final week of gestation revealed a decrease in histone 3 lysine 14 acetylation (H3K14Ac) and an increase in H3K9me2 in skeletal muscle. As a result, Glut4 expression in skeletal muscle of adult offspring is repressed (Raychaudhuri et al., 2008). Similarly, another study of rat offspring showed in uter undernutrition during gestation to decrease levels of histone 3 lysine 4 dimethylation (H3K4me2) and increase histone 3 lysine 4 trimethylation (H3K4me3) at the insulin growth factor 1 (IGF-1) locus in the liver. These IUGR offspring can undergo rapid catch-up growth and develop higher risks of metabolic syndrome and obesity. Interestingly, offspring exhibiting this phenomenon have similar epigenetic modification (i.e. at the IGF-1 locus in the liver) as those subjected to in utero undernutrition, which may contribute to an increment in liver and body weight. As such, the mechanistic relationship between IUGR and histone methylation can help to predict the risk of disease development (Tosh et al., 2010). We have found that mice fasted long term for 16 daily or 24 hours on alternate days exhibit changes to H3K9me3, leading to robust transcriptomic changes and metabolic switching within the cerebellum (data yet to be published). Nutritional stress also enhances phosphorylation of histone 3 threonine 11 (H3T11) to modulate expression of stress-response genes that influence lifespan (Oh et al., 2018). Deletion of an ATP-dependent chromatin remodeling enzyme complex imitation-switch 2 (ISW2) in yeast leads to effects that mirror CR. Yeast with ISW2 deletion exhibit increased lifespan, and upregulated stress-response, and genotoxic stresses. Overall, ISW2 appears to play important roles in mediating CR-induced effects in yeast in a mechanism distinct from suppression of TOR signaling during CR (Dang et al., 2015). Overall, DR clearly has robust effects on a plethora of histone modifications and remodeling, which may then regulate subsequent downstream effectors to mediate responses commonly reported during DR.
4.0 Dietary Restriction and microRNAs
miRNA-related research has garnered increasing interest due to its implications in aging (Jin Jung and Suh, 2012; Thalyana and Slack, 2012). The C. elegan has been a robust model organism used in the study of miRNAs during aging due to its relatively short lifespan, suitability for mutational studies, and extensive knowledge of miRNA gene libraries. Many miRNAs have been shown to be upregulated or downregulated to respectively promote or reduce lifespan in C. elegans, highlighting the relative importance of these molecular players to influence aging. Coupled with advancement in sequencing tools, a plethora of differential miRNAs have been identified and shown to be linked to aging in various tissues and organisms (Ibanez-Ventoso and Driscoll, 2009; Lencastre et al., 2010).
DR can also elicit differential changes in expression of age-related miRNAs. In C. elegans, DR induces the expression of miR-71 and miR-228. miR-228 can then repress both defective pharynx development (PHA-4) (an ortholog of human FOXA3 transcription factor) and skinhead (SKN-1) transcription factor, whereas miR-71 can repress only PHA-4. As a result, the actions of both miRNAs transduce the effects of DR to longevity in C. elegans (Sierra et al., 2015). Alterations in miRNAs may partially contribute to the neuroprotective effects commonly reported during CR. During aging in mice, there is an increase in miR-181a-1, miR-30e, and miR-34a in the brain, resulting in reduced expression of the Bcl-2 gene involved in apoptosis. CR counteracts the age-dependent increase in these miRNAs, and correspondingly increases expression of Bcl-2, decreasing apoptosis and contributing to neuroprotection and neuronal survival (Khanna et al., 2011). miR-98-3p is reportedly altered by CR in the rat cerebral cortex, altering both HDAC and histone acetyltransferase (HAT) activities (Wood et al., 2015).
CR can increase both global and mitochondrial-specific miRNAs in the liver, the most abundant being miR-122, which is critical for mitochondrial translation and induction of mitochondrial unfolded protein response, thereby improving mitochondrial proteotasis (Zhang et al., 2019). Moreover, miR-125a-5p is upregulated in the mouse liver during CR, preventing age-associated decreases. Consequently, the downstream target genes (signal transducer and activator of transcription 3 (Stat3), caspase 2 (Casp2), and StAR-related lipid transfer domain protein 13 (Stard13) are downregulated and may contribute to delayed aging (Makwana et al., 2017).
Deep sequencing has deciphered circulating serum miRNAs in young, old, and CR mice. Besides establishing that certain miRNAs are associated with aging, novel miRNAs have been discovered using this method. Again, CR can oppose age-related alterations in miRNAs to influence biological pathways implicated in aging, such as cellular metabolic pathways, Wnt signaling pathway, and apoptosis (Dhahbi et al., 2013). In rhesus monkeys, CR induces a robust change in circulating miRNAs, some of which are correlated with bodyweight, adiposity, and insulin response. miR-125a-5p was downregulated during CR, correlating positively with adiposity and negatively with insulin sensitivity (Schneider et al., 2017). miR-451, miR-144, miR-18a, and miR-15a are upregulated, whereas miR-181a and miR-181b are downregulated in skeletal muscle of old rhesus monkey, but following CR, levels of miR-181a are rescued, and the age-associated increases in miR-451 and miR-144 are prevented. Notably, expression of miRNAs in old monkeys subjected to CR resembles that seen in young animals (Mercken et al., 2013). Clearly, there is strong evidence that the effects of DR on numerous homeostatic mechanisms, critical for delaying aging, are tightly linked to the regulation miRNAs.
It is also important to consider whether the miRNA processing machinery may be impacted by DR, which would certainly impact the profile of downstream changes in expression of miRNAs. Dicer has been shown to decline with age in C. elegans, and defective Dicer results in decreased lifespan and stress tolerance. A similar phenomenon has been reported in adipose tissue of mice, with mice in which Dicer is deleted displaying hypersensitivity to oxidative stress. In both organisms, CR can counteract such phenotypes and play a key role in maintaining adipose homeostasis (Mori et al., 2012). IF has been shown to upregulate RISC components, such as Argonaute and GW-182, as well as DRSH-1 (ortholog of Drosha in Drosophila) in C. elegans. Upregulation of the processing machinery for these miRNAs has the effect of modulating the expression of target genes, such as DAF-16, the insulin/IGF-1 signaling player, which plays an important role in IF-induced longevity in C. elegans. Thus, it is important to consider the upstream processing pathway of miRNAs in response to DR as a determinant of differential downstream production of miRNAs that can effect distinct functions (Kogure et al., 2017).
A recent finding has established an interesting link between miRNAs and disease development during DR (Maniyadath et al., 2019), in that anticipatory hepatic miRNAs expressed during feeding help to counteract a fasting response. The coordinated transition between the fed-fast period is mediated by the RISC association between miRNAs and the transcriptome in the liver, which governs a plethora of homeostatic processes (such as metabolic and mitochondrial homeostasis). This biological oscillation may become dysregulated during aging, and the inability of this oscillator may result in metabolic derangements and disease development (Maniyadath et al., 2019). Furthermore, during mammary tumorigenesis in rats, CR can decrease and normalize the expression of miR-200a, which is linked to tumor progression (Devlin et al., 2016). These findings provide a strong rationale to search for either DR-related pharmacological mimetics or antagonists of miRNAs to protect against disease development.
5.0 Conclusion
The discovery of DR impacting both health span and lifespan across different organisms have driven the renaissance of nutrition research in countering aging and its associated diseases. However, the nature of DR-induced effects is complex, often varied and non-translatable between different organisms. As a result, it is highly plausible that DR possess an epigenetic milieu, which can help to explain this asymmetric manifestation of DR effects. We have outlined evidence that different types of DR regimen can distinctly modulate DNA methylation, histone modification, histone remodeling, and microRNA activity in an extremely complex, but typically beneficial, spatial, and temporal-dependent manner. Nevertheless, it seems certain that ongoing study of how DR can modulate the epigenomic axis not only can provide a better understand of underlying mechanisms, but also allow us to predict the differential effects produced by DR in different settings and to develop new types of preventive and interventional therapy to promote the duration of human health and lifespan.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Singapore National Medical Research Council Research Grant (NMRC-CBRG-0102/2016) supported this work.
References
Gavin Yong-Quan Ng1
1Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
David Yang-Wei Fann1
1Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Dong-Gyu Jo2
2School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea.
Christopher G. Sobey3
3Department of Physiology, Anatomy & Microbiology, School of Life Sciences, La Trobe University, Bundoora, Victoria, Australia.
Thiruma V. Arumugam1,2,3
1Department of Physiology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 2School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea. 3Department of Physiology, Anatomy & Microbiology, School of Life Sciences, La Trobe University, Bundoora, Victoria, Australia.
Corresponding author:
Thiruma V. Arumugam
Email: phstva@nus.edu.sg

In a new window | Download PPT
Figure 1: Dietary Restriction and Epigenetics. Schematic diagram showing robust systemic effects exerted by DR, as well as an overview of the different epigenetic mechanisms. Establishing the relationship between DR and epigenetics is critical in deciphering the epigenetic milieu of DR in producing differential responses. This may help to explain the asymmetric manifestation of DR effects, as well as allowing us to better understand the epigenetic regulation of DR by which its numerous health benefits are achieved.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10154 | 55 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA