International bi-monthly journal of cell signaling, tissue protection, and translational research.
Long Noncoding and Circular RNAs as therapeutic targets in myocardial and cerebral ischemia/reperfusion injury
Eleftheria Galatou1, Antigone Lazou2
Author Affiliations


- 1Department of Life and Health Sciences, University of Nicosia, Nicosia 2417, Cyprus.
- 2School of Biology, Aristotle University of Thessaloniki, Thessaloniki 54124, Greece.
Abstract
Acute myocardial infarction (AMI) and ischemic stroke remain two of the major causes of morbidity and mortality worldwide. Extensive research efforts have focused on the discovery or improvement of therapeutic targets and strategies to attenuate brain and heart injury after ischemia. Long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) are endogenous molecules that play key roles in the pathophysiology of cerebral ischemic stroke and myocardial infarction and are implicated in the neuronal and cardiac cell death in reperfusion injury. In this review, we summarize the latest research for linear and circular lncRNAs in myocardial and cerebral ischemia/reperfusion (I/R) injury, focusing on the role of specific lncRNAs that may promote angiogenesis or underlie cell death including apoptosis, necrosis, and autophagy. Pharmacological modulation of these noncoding RNAs could serve as a therapeutic strategy to improve clinical outcomes of patients after AMI or ischemic stroke.
Keywords: LncRNAs, circRNAs, cerebral I/R injury, myocardial I/R injury, cell death, angiogenesis
Abstract
Acute myocardial infarction (AMI) and ischemic stroke remain two of the major causes of morbidity and mortality worldwide. Extensive research efforts have focused on the discovery or improvement of therapeutic targets and strategies to attenuate brain and heart injury after ischemia. Long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs) are endogenous molecules that play key roles in the pathophysiology of cerebral ischemic stroke and myocardial infarction and are implicated in the neuronal and cardiac cell death in reperfusion injury. In this review, we summarize the latest research for linear and circular lncRNAs in myocardial and cerebral ischemia/reperfusion (I/R) injury, focusing on the role of specific lncRNAs that may promote angiogenesis or underlie cell death including apoptosis, necrosis, and autophagy. Pharmacological modulation of these noncoding RNAs could serve as a therapeutic strategy to improve clinical outcomes of patients after AMI or ischemic stroke.
Keywords: LncRNAs, circRNAs, cerebral I/R injury, myocardial I/R injury, cell death, angiogenesis
1. Introduction
The major detrimental effects of acute myocardial infarction (AMI) and brain damage after a stroke are caused by ischemia/reperfusion (I/R) injury. The decreased blood supply to tissues and consequent lack of oxygen create an environment in which the restoration of circulation results in the generation of reactive oxygen species (ROS), mitochondria dysregulation, intracellular calcium overload, cell death, leucocyte infiltration, inflammatory responses, and platelet accumulation (Sumii and Lo, 2002; Yellon and Hausenloy, 2007; Gorsuch, 2012; Lin et al., 2016; Schanze et al., 2019). After ischemic stroke or AMI, blood and oxygen supply is restored through the administration of thrombolytic drugs or by mechanical removal of thrombus [using stents in brain, percutaneous coronary intervention (PCI), or coronary artery bypass graft (CABG) surgery in heart]. However, cerebral reperfusion can lead to rapid opening of the blood-brain barrier (BBB) and severe brain damage and neurological dysfunction with poor outcome (Warach and Latour, 2004). Likewise, the sudden reperfusion of acutely ischemic myocardium has been associated with the following basic forms of injury: myocardial stunning with reversible systolic and diastolic dysfunction, no-reflow phenomenon, which is accompanied by increased infarct size, severe arrythmias, and reperfusion- induced cell death that counts for up to 50% of the final infarct size (Hausenloy and Yellon, 2013). As neuronal cells and cardiac myocytes are terminally differentiated, therapeutic approaches to limit cell death and stimulate angiogenesis would be beneficial. Angiogenesis, a physiological process in growth and development, promotes the formation of new vessels for delivery of nutrients and oxygen as a response to ischemic conditions, contributing to recovery of tissues at risk and limiting the infarcted zone.
Over the past years, human genome sequencing studies have improved characterization of noncoding RNAs and have revealed that many of them have important functions and epigenetic regulatory roles in tissue injury (Choudhuri et al., 2010; Taft et al., 2010; Guttman and Rinn, 2012). Based on size, noncoding RNAs can be categorized into short noncoding RNAs (sncRNAs <200 nucleotides) and long noncoding RNAs (lncRNAs >200 nucleotides up to 100 kilobases). According to their function and structure, sncRNAs can be divided into: a) functional RNAs, which play pivotal roles in transcription and translation such as t-RNAs, small nuclear RNAs (snRNAs), and ribosomal RNAs (rRNAs) and b) regulatory RNAs, which regulate differentially gene expression such as microRNAs (miRNAs), small interfering RNAs (siRNAs), and piwi-interacting RNAs (piwiRNAs) (Carthew et al., 2009; Ishizu et al., 2012; Schimmel, 2017). According to their structure, noncoding RNAs are categorized in linear and circular RNAs (circRNAs) (Esteller, 2011; Barrett and Salzman, 2016).
Accumulating evidence demonstrates that I/R injury modulates the expression of specific noncoding RNAs regulating the pathophysiology of ischemic stroke as well as AMI (Bayoumi et al., 2018; Ong et al., 2018; Heydari et al., 2019). This review summarizes the current state of knowledge regarding the therapeutic roles of lncRNAs and circRNAs and their implication in myocardial and cerebral I/R injury with a focus on cell death pathways and angiogenesis.
2. LncRNAs: structure and functions
There are several subtypes of lncRNAs with different structure and function and they are poorly conserved among different species (Pang et al., 2006). LncRNAs, localized in nucleus and cytoplasm, have been shown to play a significant role in the regulation and development of many diseases, including cardiovascular and neuronal diseases (Wapinski and Chang, 2011; Zhou et al., 2016; Shi and Yang, 2016). LncRNAs are transcribed by RNA polymerase II, similarly to mRNA, and are polyadenylated (Wu et al., 2008; Ramskold et al., 2009). They are classified into subfamilies according to their location: intergenic, intronic, sense, and antisense lncRNAs (Derrien et al., 2012; Ma et al., 2013). Based on gene regulation, they can also be classified as cis-lncRNAs that regulate adjacent genes through interaction with transcription factors or binding to the promoter and trans-lncRNAs, which regulate distant genes on the genome through chromatin modification, (Tsai et al., 2010; Chu et al., 2011) or binding to RNA polymerases (Yang et al., 2001; Nguyen et al., 2001; Ma et al., 2013). LncRNAs display several pivotal functions (Figure 1): 1) they regulate transcription through interference and chromatin remodeling (Bernstein et al., 2005); 2) they play a key role in post-transcriptional control and indirectly through modulating splicing factors (Tripathi et al., 2010) or directly by binding to mRNA sequences and blocking splicing (Beltran et al., 2008; Rintala-Maki and Sutherland, 2009); 3) they can bind to metal response elements (MREs) of specific miRNAs acting as competitive endogenous RNAs (ceRNAs) for miRNAs target genes (miRNA sponge) and inhibit target mRNA degradation (Cesana et al., 2011; Sumazin et al., 2011; Salmena et al., 2011; Tay et al., 2011); 4) they regulate translation by interacting with translation factors and ribosomes (Lin et al., 2008; Rintala-Maki and Sutherland, 2009; Parrott et al., 2011).
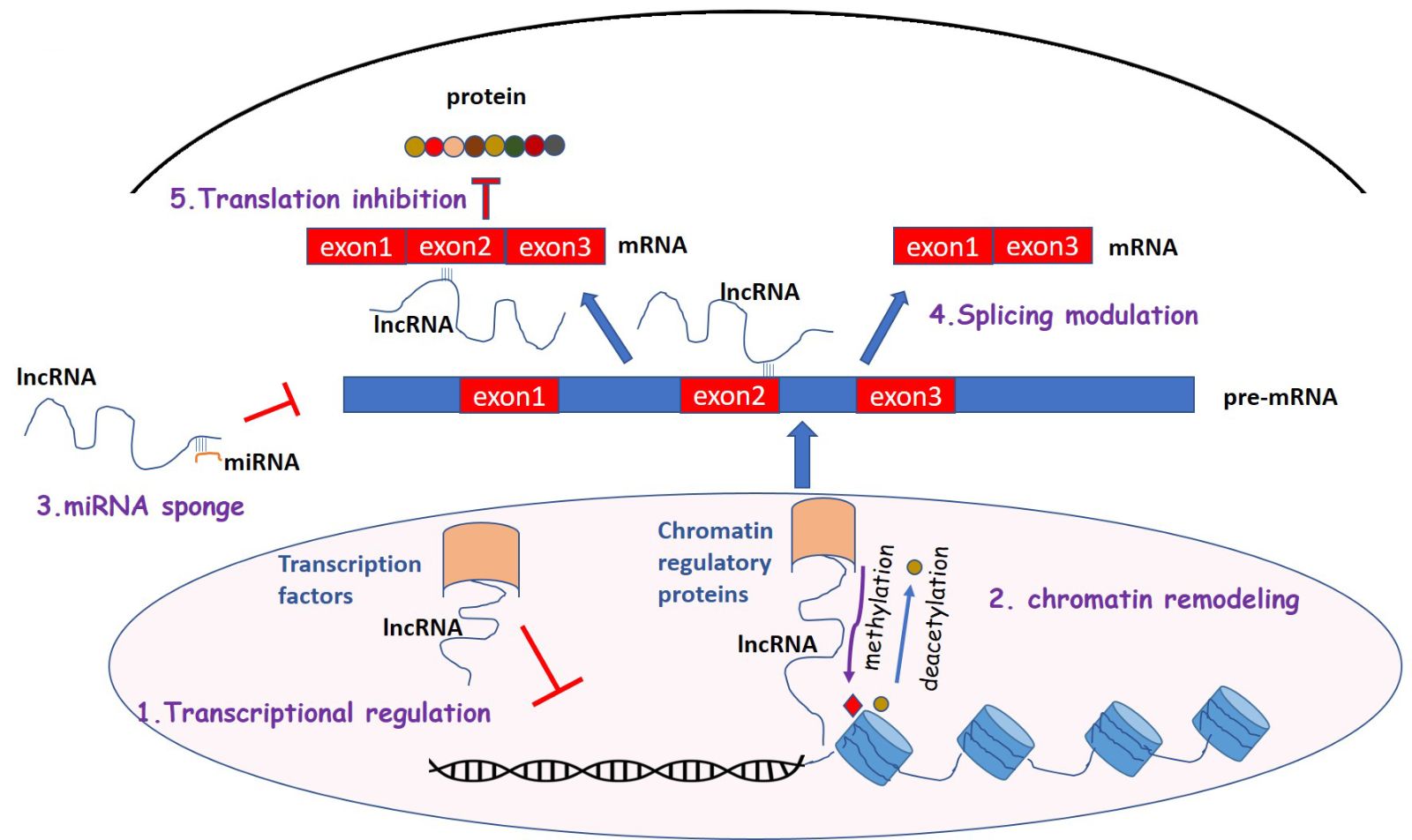
In a new window | Download PPT
Figure 1: Mechanisms of lncRNA functions. (1) Transcriptional regulation. LncRNAs interact with transcription factors and inhibit their DNA binding and transcription (2) Chromatin remodeling. LncRNAs recruit chromatin regulatory proteins and promote histone methylation and deacetylation. (3) miRNA sponges. Many lncRNAs act as competing RNAs, which interact with miRNAs and regulate the expression of miRNA target genes (4) Splicing modulation. LncRNA binds to pre-mRNA and blocks splicing or results in the formation of splicing variants. (5) Translation inhibition. LncRNAs regulate translation interacting with translation factors.
3. CircRNAs: structure and functions
CircRNAs were discovered over 20 years ago and many transcripts are abundant and stable in mammals (Jeck and Sharpless, 2014; Haque and Harries, 2017). They are transcribed by RNA polymerase II similar to linear mRNAs and they are generated by a “back splicing” process, where the 3′ OH of the 3′ exon interacts with the 5′ phosphate of the 5′ exon resulting in the formation of circRNA (Cocquerelle et al., 1993). CircRNAs are classified as exonic, intronic, antisense, and intergenic according to their genome position and play regulatory roles after binding to the promoter of host genes (Chen, 2016). CircRNAs have recently gained attention because they play a significant role in the regulation of gene expression at the transcriptional and post-transcriptional level through sponging several miRNAs (Hansen et al., 2013). CircRNAs also bind to RNA binding proteins (RBPs), which play a significant role in several cellular processes including apoptosis and angiogenesis (Ashwal-Fluss et al., 2014).
4. The role of lncRNAs and circRNAs in cerebral and myocardial I/R injury
Several lncRNAs and circRNAs have been investigated in the setting of acute ischemic stroke and AMI, and they are implicated in regulation of cell death pathways and angiogenesis. It is noteworthy that, apart from the ncRNAs that are regulated in a tissue-specific way, some of the lncRNAs are similarly regulated and exert common functions in both cerebral and myocardial I/R injury. These are highlighted below.
4.1. Cerebral I/R injury and cell death
Recent studies have demonstrated that many lncRNAs are differentially regulated in ischemic stroke and mediate cell death; these include antisense non-coding RNA in the INK4 locus (ANRIL), Fos downstream target (FosDT), small nucleolar RNA host gene 12 (SNHG12), CAMK2D-associated transcript 1 (C2dat1), and N1LR. Potential mechanisms underlying their involvement in ischemia-induced cell death include regulation of signaling pathways and interaction with transcription factors that are associated with neuronal apoptosis as well as specific miRNAs. In this context, the lncRNA ANRIL was found to be upregulated in cerebral infarcted rats and induced apoptosis possibly through regulation of the NF-kB signaling pathway (Zhao et al., 2019). The lncRNA FosDT was upregulated in middle cerebral artery occlusion (MCAO) rats after focal ischemia promoting ischemic brain damage, while FosDT silencing led to alleviation of ischemic injury. The proposed mechanism of action was linked to interaction of FosDT with coREST and Sin3a, the corepressors of repressor element-1 silencing transcription factor (REST), and with chromatin-modifying proteins (CMPs) (Mehta et al., 2015). Moreover, C2dat1 and N1LR are recently identified lncRNAs, which enhance neuronal survival in murine oxygen-glucose deprivation and re-oxygenation (OGD/R) neuronal cells through modulation of the expression of Calcium/calmodulin–dependent protein kinase II delta (CaMKIIδ) and inhibition of p53 phosphorylation respectively (Xu et al., 2016; Wu Z et al., 2017).
Accumulating evidence demonstrates that autophagy, essential for cellular homeostasis, is implicated in cerebral injury and an increasing number of lncRNAs have been shown to upregulate autophagy under these conditions. SNHG12 alleviated brain injury and cell death through upregulation of AMP-activated protein kinase (AMPK) signaling pathway, Sirtuin 1 (SIRT1) expression levels, and autophagy in MCAO mice (Yao et al., 2019; Yin et al., 2019). On the other hand, upregulation of autophagy by the lncRNA H19 was associated with increased cerebral ischemia reperfusion injury and neuronal cell death (Wang J et al., 2017). Thus, it remains controversial whether increased autophagy is neuroprotective or detrimental.
Besides regulating signaling pathways, several other lncRNAs modulate cerebral I/R injury by sponging specific miRNAs and thus regulating the expression of miRNA target proteins. Silencing of Gm11974 lncRNA attenuated cell death in OGD-treated N2a cells by modulating miR-766-3p (Cai et al., 2019). Overexpression of HOXA transcript at the distal tip (HOTTIP) lncRNA led to increased neuronal survival by inhibiting miR-143 and derepressing its endogenous target hexokinase 2 in MCAO mice and OGD- treated primary cortical neuron cells (Wang Y et al., 2018). LncRNA AK038897 sponged miR-26a-5p and upregulated death-associated protein kinase 1 (DAPK1), which is implicated in ischemic cell death (Wei et al., 2019).
Numerous circRNAs are highly expressed in the central nervous system and are implicated in the regulation of several processes. Bai et al. (2018) demonstrated the neuroprotective role of circRNA DLGAP4 in BBB permeability and brain injury in MCAO mice and patients after an ischemic stroke, by sponging miR-143. On the other hand, the circRNAs HECT domain E3 ubiquitin protein ligase 1 (HECTD1) and TLK1 exert detrimental effects on cerebral ischemic injury by promoting astrocyte autophagy through inhibition of miR-142 (Han et al., 2018) or by modulating 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible poly (ADP-ribose) polymerase (TIPARP) through sponging miR-335-3p, respectively (Wu et al., 2019). Moreover, Liu et al. (2019) detected a number of circRNAs, which are differentially expressed in OGD/R- treated primary brain microvascular endothelial cells (BMEC) and sponge several miRNAs. They also revealed that most of the detected circRNAs are implicated in the regulation of calcium ion related- and nitric oxide (NO)/cyclic guanosine 3′,5′-monophosphate (cGMP) signaling pathways, which play pivotal role in cerebral I/R injury.
In conclusion, lncRNAs and circRNAs promote or alleviate cerebral I/R- induced cell death by regulating several signaling pathways and sponging miRNAs and may be promising therapeutic targets for the treatment of ischemic stroke.
4.2 Myocardial I/R injury and cell death
Many lncRNAs can stimulate or inhibit cell death in ischemic heart by sponging specific miRNAs and/or by regulating related signaling pathways. Zinc finger antisense 1 (ZFAS1), a cardiac-specific lncRNA, is overexpressed in AMI and promotes cell death and myocardial injury via downregulation of miR-150 and activation of C- reactive protein (CRP) (Wu T et al., 2017). Regulator of reprogramming (ROR) and KQT-like subfamily, member 1 opposite strand/antisense transcript 1 (KCNQ1OT1) lncRNAs are highly expressed in patients with I/R injury and in hypoxia-reperfusion-treated cardiomyocytes and lead to increased expression levels of proapoptotic genes through regulation of p38 mitogen-activated protein kinase (MAPK) (Zhang W et al., 2018) and nuclear factor-kB (NF-kB) signaling pathways (Li X et al., 2017). Suppression of RNA component of mitochondrial RNA processing endoribonuclease (RMRP), in an in vivo rat model of myocardial I/R injury, improved cardiac function and inhibited apoptosis after myocardial I/R injury, by modulating miR-206 (Kong et al., 2019). Furthermore, in hypoxia-treated H9C2 cells, lncRNA tumor associated long non-coding RNA expressed on chromosome 2 (TALNEC2) has been found to aggravate hypoxia injury by regulating miR-21/programmed cell death protein 4 (PDCD4)-mediated activation of the Wnt/β-catenin pathway (Hao et al., 2019). LncRNAs have also been associated with necrosis. Necrosis related factor (NRF) lncRNA is regulated by transcription factor p53 and promotes necrosis in myocardial I/R injury through repression of miR-873 (Wang et al., 2016).
CircRNAs also play pivotal roles in I/R-induced cardiomyocyte death. Besides their presence in the cytoplasm and nucleus, circRNAs are abundant in whole blood, plasma, and extracellular vesicles and they are resistant to degradation by exoribonucleases, making them potential biomarkers for diagnosis and treatment of AMI (Devaux et al., 2017; Gomes et al., 2018). Recent studies demonstrated alterations in the expression profile of circRNAs in patients with ΑMI. Myocardial infarction-associated circRNA (MICRA) was detected in peripheral blood cells of > 600 patients with AMI in two independent cohorts. MICRA was associated with left ventricular (LV) remodeling confirming its predictive value for ΑMI (Salgado-Somoza et al., 2017). Moreover, 185 circRNAs were differentially expressed in extracellular vesicles isolated from I/R- treated murine hearts (Ge et al., 2019). Upregulated circRNAs were associated with signaling through erythropoietin-producing hepatocellular carcinoma receptor (Eph) and their interacting ligands (Ephrins), and are implicated in I/R- induced apoptosis. On the other hand, downregulated circRNAs were associated with SMAD signaling, fibrosis, and cardiac dysfunction after I/R injury. Other studies have revealed that the circRNAs circ_101237, MFACR, and circNCX1 target specific miRNAs to enhance heart dysfunction and promote mitochondrial fission and apoptosis (Wang K et al., 2017; Li et al., 2018; Gan et al., 2020). Furthermore, silencing of circ_0010729 in OGD-treated human cardiomyocytes attenuated apoptosis, inhibiting BAX and cleaved caspase 3 and 8 expression levels (Jin and Chen, 2019). Silencing of this circRNA eliminated hypoxia-induced injury through upregulation of mTOR and MEK/ERK pathway by regulating miR-145-5p.
Apart from apoptosis and necrosis, there is growing evidence that specific lncRNAs and circRNAs regulate autophagy in myocardial injury. The lncRNAs APF, GATA1 activated lncRNA (Galont), AK139328, and AK088388 promote autophagy-mediated cell death through modulation of several miRNAs (Wang et al., 2015; Yin et al., 2018; Yu et al., 2018; Wang J et al., 2019). In contrast, the lncRNA CAIF and the circRNA ACR exert a cardioprotective role in AMI by suppressing autophagy-mediated cell death by modulating p53 (Liu CY et al., 2018) and upregulating Pink1 expression (Zhou et al., 2019). Finally, overexpression of the lncRNA nuclear-enriched abundant transcript 1 (NEAT1) attenuated myocardial injury-induced apoptosis and autophagy by elevating miR-181b expression levels (Lv et al., 2020).
4.3 LncRNAs in ischemic conditioning approaches
Ischemic conditioning approaches are powerful protective strategies that reduce tissue damage after I/R. Ischemic preconditioning (IPC), ischemic postconditioning (IPost), and remote ischemic conditioning (RIC) have been shown to limit myocardial infarct size after I/R and improve survival of patients (Hausenloy and Yellon, 2016; Hausenloy et al., 2016; Pavo et al., 2017). Increasing evidence has highlighted the role of miRNAs as mediators of cardioprotection by IPC, IPost or RIC (Salloum et al., 2010; Varga et al., 2014; Ong et al., 2018; Bartekova et al., 2019 ; Kura et al., 2020). However, only a few studies have illustrated the role of lncRNAs in myocardial ischemic conditioning. Chen Z et al. (2018) demonstrated that morphine induced IPost alleviated myocardial I/R injury in a rat model and upregulated the lncRNA Urothelial Carcinoma-Associated 1 (UCA1), which downregulated miR-128 and expression of autophagy markers. Furthermore, lncRNA H19 was shown to be upregulated in vitro in H2O2 preconditioning-treated H9C2 cells, in hypoxia preconditioning-treated neonatal rat cardiomyocytes, and in vivo in murine hearts subjected to IPC (Chen et al., 2020). The protective effects of LncRNA H19 overexpression on myocardial I/R injury were mediated through transcriptional and posttranscriptional regulation of nucleolin protein (Chen et al., 2020). To our knowledge, there is no information on the role of lncRNAs in the cardioprotection induced by RIC.
Accumulating evidence has reported that ischemic conditioning also exerts neuroprotective effects by improving tolerance of the brain to ischemic events and attenuating cerebral damage (Dirnagl et al., 2003; Wang et al., 2015; Li S et al., 2017). Moreover, several studies have demonstrated alterations in miRNAs profiles indicating their involvement in ischemic conditioning-induced neuroprotection (Dharap and Vemuganti, 2010; Lee et al., 2010; Miao et al., 2016; Bell et al., 2017). However, evidence on the involvement of lncRNAs in neuroprotection by ischemic conditioning is scarce and their role is yet to be established.
4.4 Noncoding RNAs with common regulation and function in cerebral and myocardial I/R injury
Some lncRNAs have common regulatory roles in cell death pathways both in cerebral and myocardial I/R injury. They target the same or different set of miRNAs and they have similar or opposing functions (Figure 2). LncRNA maternally expressed gene 3 (MEG3) is expressed in many tissues, including the brain and heart. MEG3 represents a cytotoxic factor and numerous recent studies reported that MEG3 promotes ischemia-induced neuronal and cardiac cell death through sponging several miRNAs. In the brain of MCAO mice, Liu et al. (2016) demonstrated that MEG3 knockdown upregulated miR-181b and attenuated neuronal cell apoptosis through inhibition of lipoxygenase 12/15-LOX expression. Another study showed that MEG3 competed with PDCD4 for binding to anti-apoptotic miR-21, thus inhibiting apoptosis and improving brain injury (Yan et al., 2017). In the heart, MEG3 targets different miRNAs. Meg3 silencing in I/R simulated H9C2 cells led to decreased cell death through modulation of miR-183 and p27 suppression (Gong et al., 2018), and through regulation of miR-7-5p and caspase 3 activity (Zou et al., 2019).
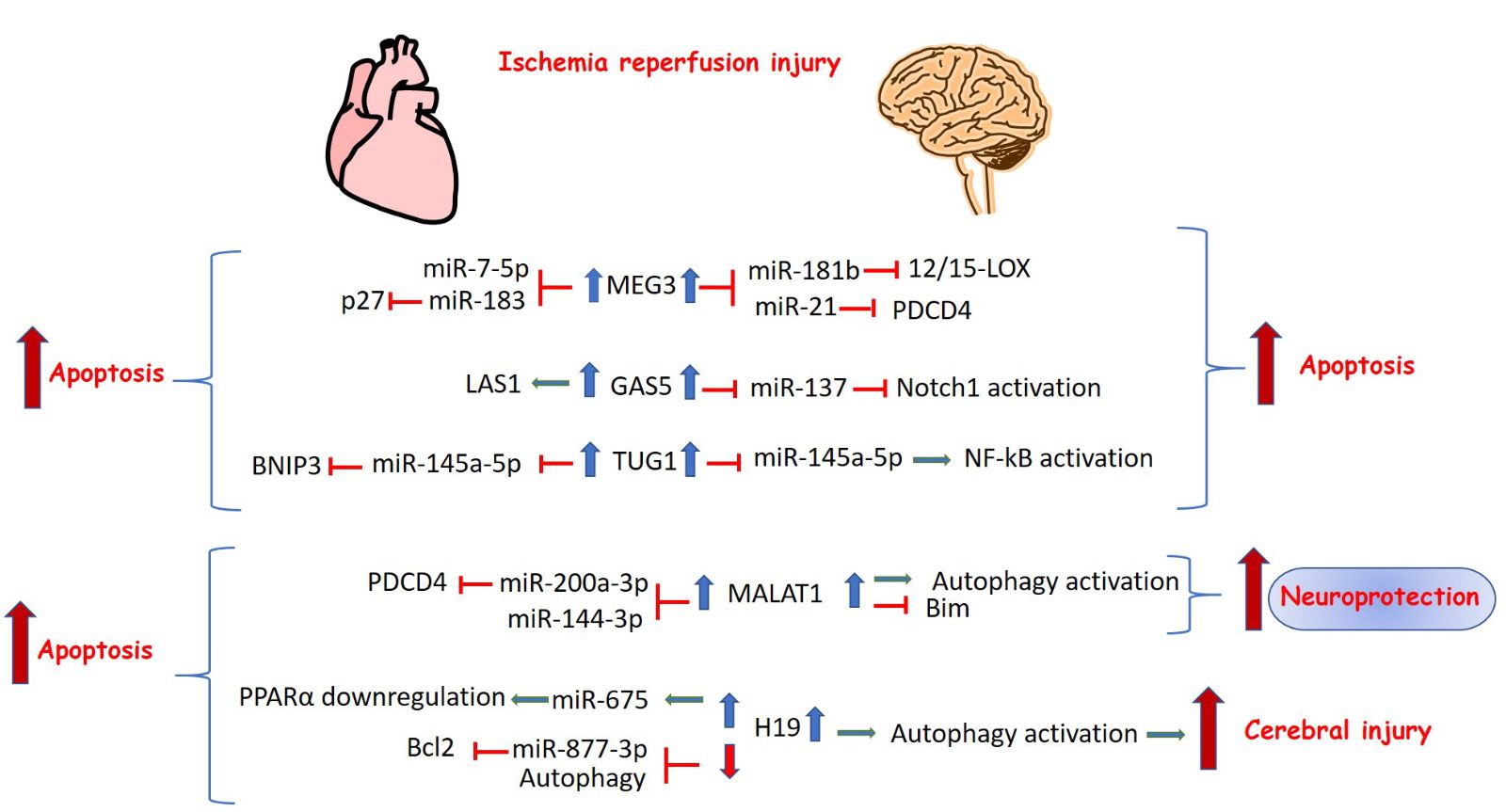
In a new window | Download PPT
Figure 2: Long noncoding RNAs with common regulation and function in cerebral and myocardial I/R injury. The upregulation of the lncRNAs MEG3, GAS5, TUG1, MALAT1, and H19 modulates cerebral or myocardial I/R-induced cell death by regulating signaling pathways and sponging specific miRNAs.
Growth arrest-specific 5 (GAS5) is another lncRNA that is implicated in the pathogenesis of ischemic injury and exerts common functions in the brain and heart. Chen F et al. (2018) demonstrated that GAS5 silencing inhibited neuronal apoptosis through modulation of miR-137 and the Notch1 signaling pathway in MCAO mice and OGD/R-treated primary mouse cortical neurons. Recently, Liu SD et al. (2018) demonstrated a similar function of GAS5 in myocardial I/R injury. GAS5 promoted apoptosis through activation of LAS1- regulator of myocardial I/R injury, modulating the p38 MAPK signaling pathway.
Furthermore, H19 also regulates apoptosis and autophagy in cerebral and myocardial I/R injury is H19. Wang J et al. (2017) reported that H19 expression was increased in I/R conditions in vitro and in vivo and its downregulation decreased I/R- induced apoptosis by regulating autophagy. Similarly, Luo et al. (2019) demonstrated that the expression levels of H19 and miR-675 were up-regulated in OGD/R-treated cardiomyocytes. Knockdown of H19, a precursor of miR-675, inhibited proapoptotic genes modulating peroxisome proliferator-activator receptor alpha (PPARα) expression. However, other studies demonstrated that H19 was downregulated in H2O2-treated cardiomyocytes and mice with I/R injury, whereas H19 overexpression attenuated apoptosis and decreased infarct size by targeting miR-877-3p and upregulating the antiapoptotic gene Bcl2 (Li et al., 2019) or by activating autophagy (Zhou et al., 2018).
As mentioned above, apart from sponging different miRNAs to modulate cell death in the brain and heart, lncRNAs may also target the same set of miRNAs in both tissues. Taurine up-regulated gene 1 (TUG1) lncRNA negatively regulated miR-145a-5p leading to NF-kB- mediated activation of the inflammatory pathway (Wang H et al., 2019). Interestingly, TUG1 sponged the miRNA miR-145a-5p and aggravated hypoxia-induced apoptosis and myocardial cell injury through upregulation of Bcl2 interacting protein 3 (BNIP3) and activation of Wnt/β-catenin signaling pathways (Wu et al., 2018). On the other hand, other lncRNAs display different functions in ischemic brain and heart. Metastasis- associate lung adenocarcinoma transcript 1 (MALAT1) lncRNA is a representative example. Although MALAT1 upregulation is associated with anti-apoptotic effects in brain ischemic injury, it seems to increase injury in the infarcted heart. Zhang X et al. (2017) found that MALAT1 upregulation decreased cell caspase 3 activity and proapoptotic gene expression in OGD-treated BMECs. Moreover, MALAT1 knock-out mice demonstrated increased cerebral infarct size and brain damage compared to controls. Other recent studies have shown that MALAT1 exerts its neuroprotective role by sponging specific miRNAs and reversing autophagy inhibition by upregulating the autophagy related gene, Unc-51-Like Kinase 2 (ULK2) (Li Z et al., 2017) and by activating the SIRT-1 pathway (Wang S et al., 2019). In contrast, MALAT1 is upregulated in patients with acute AMI, in mice subjected to left anterior descending (LAD) coronary artery occlusion as well as in cardiomyocytes after hypoxia/reperfusion and promoted apoptosis through downregulation of miR-144-3p expression (Gong et al., 2019) or through the miR-200a-3p/PDCD4 axis (Sun R et al., 2019). Moreover, ablation of MALAT1 decreased apoptotic levels in hypoxia/reoxygenation-treated murine cardiomyocytes through autophagy activation by regulating TSC2-mTOR signaling (Hu et al., 2019).
4.5 Cerebral I/R injury and angiogenesis
Recent evidence has implicated lncRNAs in angiogenesis after ischemia injury. SNHG12 lncRNA was upregulated in primary BMECs after OGD treatment and promoted cell migration, vascular endothelial growth factor (VEGF) expression, and subsequently angiogenesis by targeting miR-150 (Zhao et al., 2018) or miR-199a (Long et al., 2018; Wang Z et al., 2018). Other studies reported that knockdown of lncRNA Meg3 led to improvement of infarct size and neuronal survival, and promoted angiogenesis via upregulation of the Notch pathway in MCAO rats (Liu et al., 2017) or activation of the Wnt/β-catenin signaling pathway (You D and You H, 2019). Moreover, Zhan et al. (2017) demonstrated that lncRNA MEG3 inhibition protected rat BMEC cells against OGD/R-induced apoptosis through downregulation of NADPH oxidase 4 (NOX4) and p53, and enhanced pro-angiogenic factor (HIF-1α and VEGF) expression. The lncRNA ANRIL was also associated with angiogenesis in cerebral infarction. Overexpression of ANRIL in diabetic rats combined with cerebral infarction increased VEGF expression and microvessel density, and enhanced angiogenesis through activation of the NF-kB pathway (Zhang B et al., 2017). Therefore, targeting angiogenesis is an important strategy that confers neuroprotection by restoring blood supply and alleviating brain injury after ischemic stroke. lncRNAs appear to be significant mediators of angiogenesis in experimental models of cerebral I/R, thus, they could serve as potential neuroprotective strategies against ischemic stroke.
4.6 Myocardial I/R injury and angiogenesis
Evidence on the role of lncRNAs in angiogenesis after myocardial ischemic injury is limited. It has been reported that the lncRNA C2dat1 attenuated hypoxia-induced injury and elevated VEGF expression levels through sponging miR-22 (Sun H et al., 2019). Overexpression of lncRNA myocardial infarction-associated transcript (MIAT) led to downregulation of miR-150-5p, which in turn increased VEGF levels and promoted angiogenesis in retinal endothelial cells (Yan et al., 2015). Moreover, overexpression of circRNA circFndc3b decreased cardiomyocyte apoptosis, improved cardiac function, and enhanced angiogenesis after AMI in mice by upregulating VEGF-A (Garikipati et al., 2019). Finally, Zhang et al. (2020) demonstrated that knockdown of circRNA hsa_circ_0007623 in hypoxia-induced human umbilical vein endothelial cells (HUVEC) decreased cell proliferation, migration, and angiogenesis via modulation of the miR-297/VEGF axis. Lentivirus-induced overexpression of hsa_circ_0007623 in isoproterenol-induced ischemic mice improved cardiac function and promoted VEGF expression (Zhang et al., 2020).
Further exploration of more lncRNAs and circRNAs involved in angiogenesis may define the exact mechanisms of action and their role in cerebral and myocardial I/R injury.
5. Clinical implications
Clinical studies have demonstrated that several lncRNAs are dysregulated in patients with ischemic stroke and AMI and could be used as diagnostic/prognostic markers. Dykstra-Aiello et al. (2016) reported changes in expression of 299 lncRNAs and 97 lncRNAs in blood samples of male and female stroke patients compared with the corresponding control subjects. The authors also reported that certain lncRNAs changed expression over time following stroke, suggesting their potential as diagnostic biomarkers. Moreover, in a recent clinical study it was demonstrated that ischemic stroke changed the expression of specific lncRNAs over time in peripheral blood mononuclear cells of ischemic stroke patients and highlighted the role of lncRNAs in the peripheral immune system (Zhu W et al., 2019). Wang J et al. (2017) demonstrated that specific polymorphisms in H19 gene increased the risk of stroke in ischemic patients. In addition, another clinical study demonstrated the detrimental effect of circRNA HECTD1 in the development and progression of acute ischemic stroke. CircRNA HECTD1 expression levels were elevated in peripheral blood samples of 160 ischemic patients compared with 160 controls and predicted higher recurrence risk of acute ischemic stroke (Peng et al., 2019). Finally, Zhu X et al. (2019) evaluated the expression levels of circRNA DLGAP4 and pro-inflammatory miR-143 in peripheral blood mononuclear cells of patients with acute ischemic stroke. The authors revealed that circRNA DLGAP4 was downregulated in ischemic patients and it was negatively correlated to miR-143 and inflammation markers, supporting their value in the prediction of acute ischemic stroke risk.
In patients with AMI, Vausort et al. (2014) demonstrated differential expression profiles of five lncRNAs (aHIF, ANRIL, KCNQ1OT1, MIAT, and MALAT1) in blood cells. Alterations in the expression levels of circulating lncRNAs UCA1, Cdr1 antisense (CDR1AS), and ZFAS1 were also reported in the setting of AMI (Yan et al., 2016; Zhang et al., 2016). Although further research is needed to define the exact role of lncRNAs in myocardial injury, the above clinical studies support the prognostic value of lncRNAs and provide new venues for early prognosis of AMI.
6. LncRNAs as therapeutic targets
Given that dysregulation of a number of lncRNAs and circRNAs are observed in AMI and stroke, therapeutic inhibition or activation of these ncRNAs may provide a potential therapeutic strategy for cardioprotection and neuroprotection respectively.
Silencing of targeted RNA molecules can be achieved by the use of antisense oligonucleotides (ASO) and siRNAs. Taken into consideration that lncRNAs have nuclear or cytoplasmic localization, and many of them are tissue specific, different delivery methods could be used for improvement of ischemic injury (Bennett et al., 2017; Lucas et al., 2018; Smith and Zain, 2019). This needs to be considered for the general targeting strategy. siRNAs target mainly cytoplasm localized RNAs (Lennox and Behlke, 2016), whereas GapmeRs, the most efficient class of ASOs, downregulate genes inside the nucleus. Since most lncRNAs are localized in the nucleus, GapmeRs represent a very promising tool for pharmacological silencing of lncRNAs.
On the other hand, overexpression of lncRNAs can be achieved with the use of adeno-associated viral (AAV) vectors, lentiviruses, nanoparticles, exosomes, and also RNA mimics. However, the large size of lncRNAs that impedes their delivery through the BBB is an important challenge to be addressed. Exosomes and their mimetic delivery systems (liposomes) provide many advantages in targeting specific tissue and represent efficient delivery strategies that could facilitate passage through the BBB and also improve the bioavailability of lncRNAs to ischemic brain and cardiac tissues (Lakhal and Wood, 2011; Ong et al., 2017; Sluijter et al., 2018). However, although high-throughput sequencing technologies combined with bioinformatics have enlightened the field of transcriptomics, several limitations should be resolved before translating this therapeutic approach of lncRNAs into the clinical setting (Bassett et al., 2014). Therefore, there is crucial need for a collective effort to elucidate the complex role of the transcriptome in ischemic diseases. Along these lines, the CardioRNA European Cooperation in Science and Technology (COST) Action CA17129 aims to strengthen the understanding of ncRNAs in cardiovascular diseases, providing a network for collaboration between relevant researchers and clinicians to transfer the knowledge into translational research and personalized medicine (Gomes et al., 2019).
7. Conclusions
The current strategy for the treatment of patients with acute ischemic stroke include the thrombolytic agent tissue plasminogen activator (r-tPA), but the narrow therapeutic window limits its use (Group I-C, 2012; Tan et al., 2014). On the other hand, although thrombolytic therapy and primary percutaneous coronary intervention (PPCI) improves injury after AMI, there are still no effective strategies for cardioprotection. Thus, new therapeutic targets are required to protect the heart and brain from the detrimental effects of I/R injury. An increasing number of lncRNAs and circRNAs have been identified and play significant regulatory roles in the setting of AMI and ischemic stroke. Noncoding RNAs, including lncRNAs and circRNAs modulate the expression levels of target genes and downstream signaling pathways either directly through regulating RNA splicing and RNA degradation, or indirectly by affecting miRNA functions and acting as competing endogenous RNAs. Several studies demonstrated that cerebral and myocardial I/R injuries are associated with alterations in the expression levels of specific lncRNAs, which act as promoters or suppressors of cell death and angiogenesis. Thus, blocking or overexpression of specific lncRNAs in vivo could be an effective therapeutic strategy to treat ischemic injuries. Based on their functions and the underlying mechanisms, lncRNAs may hold the potential to improve patient outcomes and to promote cardiac and brain regeneration to limit infarct size and repair injured tissue. Taken together, lncRNA-based therapies represent promising strategies and tools for the treatment and also prevention of ischemic stroke and AMI.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements:
This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
Eleftheria Galatou1
1Department of Life and Health Sciences, University of Nicosia, Nicosia 2417, Cyprus.
Antigone Lazou2
2School of Biology, Aristotle University of Thessaloniki, Thessaloniki 54124, Greece.
Corresponding author:
Eleftheria Galatou
Email: galatou.e@unic.ac.cy

In a new window | Download PPT
Figure 1: Mechanisms of lncRNA functions. (1) Transcriptional regulation. LncRNAs interact with transcription factors and inhibit their DNA binding and transcription (2) Chromatin remodeling. LncRNAs recruit chromatin regulatory proteins and promote histone methylation and deacetylation. (3) miRNA sponges. Many lncRNAs act as competing RNAs, which interact with miRNAs and regulate the expression of miRNA target genes (4) Splicing modulation. LncRNA binds to pre-mRNA and blocks splicing or results in the formation of splicing variants. (5) Translation inhibition. LncRNAs regulate translation interacting with translation factors.

In a new window | Download PPT
Figure 2: Long noncoding RNAs with common regulation and function in cerebral and myocardial I/R injury. The upregulation of the lncRNAs MEG3, GAS5, TUG1, MALAT1, and H19 modulates cerebral or myocardial I/R-induced cell death by regulating signaling pathways and sponging specific miRNAs.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10125 | 27 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA