Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Role of temperature in myocardial ischemic injury and protection by conditioning
Time:2020-02-29
Number:12925
Pasquale Pagliaro1, Manuela Aragno1, Claudia Penna1
Author Affiliations


- 1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy.
Conditioning Medicine 2020. 3(1): 31-46.
Abstract
Organ temperature and ischemia/reperfusion injury (IRI) are strictly linked. It is well known that deep/profound hypothermia can improve cardioplegia induced cardiac preservation. Also mild/moderate hypothermia induces myocardial protection against IRI in beating hearts. Here we will focus on some mechanisms of cardioprotection induced by hypothermia. In particular, we will analyze the effects of hypothermia on heart metabolism, cytosolic calcium, and oxidation/nitrosation processes. The role of inflammation, extracellular matrix processing, genetic, and epigenetic effects in mild hypothermia will be also analyzed. Recent advances on miRNA, exosomes, and extracellular vesicles controlled by temperature variation will be discussed. Particular emphasis will be given to the procedures of conditioning (pre, per and postconditioning and remote conditioning) obtained with thermic and/or ischemic stimuli. In this context hyperthermic processes also play an important protective role. From the analyzed studies, it emerges that temperature is an important variable affecting IRI that deserves to be strictly controlled and eventually modified to amplify the efficacy of cardioprotective strategies. Nevertheless, more in depth studies are necessary to understand the mechanisms of protection induced by thermic therapy alone and in association with other cardioprotective interventions. More in depth investigation into the mechanistic signaling that lead to temperature triggered protection could individuate crucial targeting molecules, representing strategic knowledge to promote a better therapeutic efficacy and a lower cardiovascular risk in the setting of myocardial ischemia/reperfusion.
Abstract
Organ temperature and ischemia/reperfusion injury (IRI) are strictly linked. It is well known that deep/profound hypothermia can improve cardioplegia induced cardiac preservation. Also mild/moderate hypothermia induces myocardial protection against IRI in beating hearts. Here we will focus on some mechanisms of cardioprotection induced by hypothermia. In particular, we will analyze the effects of hypothermia on heart metabolism, cytosolic calcium, and oxidation/nitrosation processes. The role of inflammation, extracellular matrix processing, genetic, and epigenetic effects in mild hypothermia will be also analyzed. Recent advances on miRNA, exosomes, and extracellular vesicles controlled by temperature variation will be discussed. Particular emphasis will be given to the procedures of conditioning (pre, per and postconditioning and remote conditioning) obtained with thermic and/or ischemic stimuli. In this context hyperthermic processes also play an important protective role. From the analyzed studies, it emerges that temperature is an important variable affecting IRI that deserves to be strictly controlled and eventually modified to amplify the efficacy of cardioprotective strategies. Nevertheless, more in depth studies are necessary to understand the mechanisms of protection induced by thermic therapy alone and in association with other cardioprotective interventions. More in depth investigation into the mechanistic signaling that lead to temperature triggered protection could individuate crucial targeting molecules, representing strategic knowledge to promote a better therapeutic efficacy and a lower cardiovascular risk in the setting of myocardial ischemia/reperfusion.
1. Introduction
Animal species that maintain a body temperature higher than their environment are commonly called “warm-blooded animals.” Among these are birds and mammals, homoeothermic species that maintain a stable body temperature by adjusting metabolic processes. In homoeothermic species, the internal temperature (core temperature) is often higher than the immediate environment (Zivadinović et al., 2005; Mitchell et al., 2018; Romanovsky 2018).
Metabolic process that generates heat energy are many, among these muscle contraction is one of the most efficient. Therefore, during manual work and physical exercise, muscle and internal body temperature may be a little higher than the typical core temperature of the considered animal (Gleeson 1998).
Human core temperature is 37 °C (98.6 °F) and can range between 36.1 °C (97 °F) and 37.2 °C (99 °F). Body temperature of some animals used for experiments is higher than that of humans. For example, those of dogs and pigs are between 37.5 and 39.5 °C while the murine model is similar to human (from 36.5 to 38 °C) (Ingram and Legge, 1970; Nemoto and Frankel 1970). During normal life activities, maintaining a constant core body temperature is essential for survival of these animals, by maintaining optimal organ, tissue, and cellular function.
Usually, with ischemia the temperature of the organ decreases, in particular the temperature of those organs and parts of the body exposed to room temperature. Furthermore, reperfusion exposes organs to rapid variations in temperature. In contrast, internal organs, such as the heart, may have little, if any, temperature variation during ischemia and reperfusion in situ, in the majority of clinical conditions. Yet, in some clinical (e.g. during heart surgery) or experimental conditions (e.g. open thorax or isolated heart), the heart temperature may randomly drop during ischemia. Therefore, the extent of ischemia/reperfusion injury (IRI) may be influenced by temperature variation during such conditions. In some other clinical conditions, the temperature is under the control of physicians, who try to reduce it during organ ischemia to reduce IRI.
Since temperature plays a pivotal role in determining myocardial IRI, many studies considered the role of temperature in determining myocardial infarct size. Studies also considered the role of temperature in determining the efficacy of conditioning protocols. In this mini-review we will analyze the findings of some of these studies in homoeothermic animals. Specifically, we provide the current state of knowledge regarding therapeutic hypothermia against IRI and the mechanisms of hypothermia-induced myocardial protection. Moreover, we discuss how it is possible to induce cardioprotection by combining conditioning protocols with temperature variations.
For an interesting evolutionary and comparative perspective, the reader is directed to reviews dealing with hypothermic mechanisms of hypoxia tolerance in ectotherms (Tota et al., 2011) and hibernating mammals (Dave et al., 2012). Indeed, myocardial ischemia often leads to devastating damage in humans and most other mammals, whereas ectotherms and hibernating mammals suffer no ill effects when blood flow to an organ is drastically decreased during experimentally induced ischemia in euthermia or during torpor. Such models might reveal to us some mechanisms that might be exploited in humans.
2. Temperature and ischemia/reperfusion
In mammals, myocardial IRI is a process in which several mechanisms are triggered that lead cells to death. During ischemia and reperfusion, oxidative stress and calcium overload, as well as lowering of ATP levels may reach critical levels at which the ability of the cardiac cells to remain viable is compromised, and the cells undergo uncontrolled death through processes of oncosis and necrosis. Moreover, programmed cell death pathways may be initiated including apoptosis, necroptosis, and pyroptosis. Each of these cell death types contribute to infarct size and may activate different levels of the inflammatory response (Davidson et al., 2020). In the last few years we have understood that the optimal cardioprotective approach may require the combination of synergistic or additive multi-target therapies (Davidson et al., 2019).
Temperature is among one of the most important variables to control during cardioprotective strategies to reduce cell death and inflammatory processes. Temperature plays a major role in determining infarct size in homoeothermic mammals (Chien et al., 1994; Hale and Kloner 1997; Schwartz et al., 1997; Guo et al., 2012). Indeed, during the ischemic period, infarct size is proportional to the temperature of the heart (Miki et al., 1998). Temperature must be carefully controlled throughout the experiment by lamps and heating pads, and must be continuously monitored using a stabilized rectal thermometer. For example, the heated table and thermal mattress should be warmed up to 40-42 °C to maintain the core body temperature around 37-38 °C throughout the procedures (Gao et al., 2011; Dai et al., 2015; Bøtker et al., 2018).
It seems that even in the absence of ischemia, hearts tolerate hypothermia better than hyperthermia (Hale and Kloner 1997; Tissier et al., 2012). Many studies were conducted under hypothermic conditions to assess the cardioprotective mechanism of hypothermia before, during, and after ischemia. Studies on the protective role of hyperthermia have been also carried out (see below). Indeed, hypothermia protects ischemic tissue in many organs including heart and brain, increasing the rate of a favorable outcome and reducing mortality (Dave et al., 2012; Yenari et al., 2008; Savitz et al., 2017). Actually, the protective effect of lowering temperature has been known since antiquity and hypothermia was put into surgical practice early in the 1950’s. In the Classical Greece period Hippocrates had already recognized an important role of temperature management by inducing cooling during fever (Polderman, 2004). More recently, Bigelow et al., (1950)applied therapeutic hypothermia during cardiac surgery as a form of therapeutic myocardial protection. Over the years, the effects of change in temperature have been the object of numerous studies and represent a critical avenue for development of new therapies. Even the first successful open heart operations were performed under hypothermic conditions (Lewis and Taufic, 1953). After the early applications of hypothermia for brain protection during aortic arch repair, this technique has been exploited worldwide in aortic surgery (Griepp et al., 1975).
In the cardiology field, besides infarct size reduction, mild hypothermia also results in prevention of no-reflow or microvascular obstruction, preservation of post-ischemic contractile function, and ultimately improvement in cardiac remodeling in several animal studies (Tissier et al., 2012). However, previous large clinical studies applying hypothermia in patients with ST-segment elevation myocardial infarction (STEMI) were disappointing (Grines, 2004). This discrepancy between animals and humans could be due to many factors, including technical problems that have not made it possible to significantly reduce normothermic ischemic times in humans. Indeed, more recent trials in which rapid cooling was achieved seems more promising in terms of infarct size reduction and/or limiting the severity of post-ischemic heart failure (Götberg et al., 2010; Erlinge et al., 2013, 2014, 2015; Noc et al., 2017) (Table 1). Much larger studies are needed to verify these results in light of the fact that lowering the temperature below 30 °C is dangerous and can jeopardize life. Nevertheless, these promising trials are a stimulus to better understand the effects of temperature in myocardial protection to more safely exploit these procedures.
3. Mechanisms of mild/moderate hypothermia-induced myocardial protection
Hypothermia can be classified based on the depth of cooling. Therefore, we can have mild hypothermia (32-35 °C), moderate hypothermia (28-32 °C), deep hypothermia (28 to 17 °C), and profound (<17 °C) hypothermia. Mild/moderate hypothermia may be cardioprotective and can reduce infarct size in several animal species (e.g. dogs, pigs, rabbits, sheep, and rats) (Tissier et al., 2012). Deep/profound hypothermia has been widely used to improve the outcome in many clinical conditions, including cardiac arrest, transplantation, hemorrhagic shock, and myocardial ischemia.
The mechanisms underlying hypothermia-induced protection of the brain and heart have been studied since the early 1950s (Thorn et al., 1957; Kübler, 1967). They have been analyzed in recent reviews (e.g. Hale and Kloner, 2011; Tissier et al., 2012). Therefore, herein we analyze some among the most common and recently described mechanisms. Indeed, mild/moderate hypothermia does not act only via energy saving, it acts also through intracellular signaling pathways, intercellular communication and other mechanisms (Fig 1).
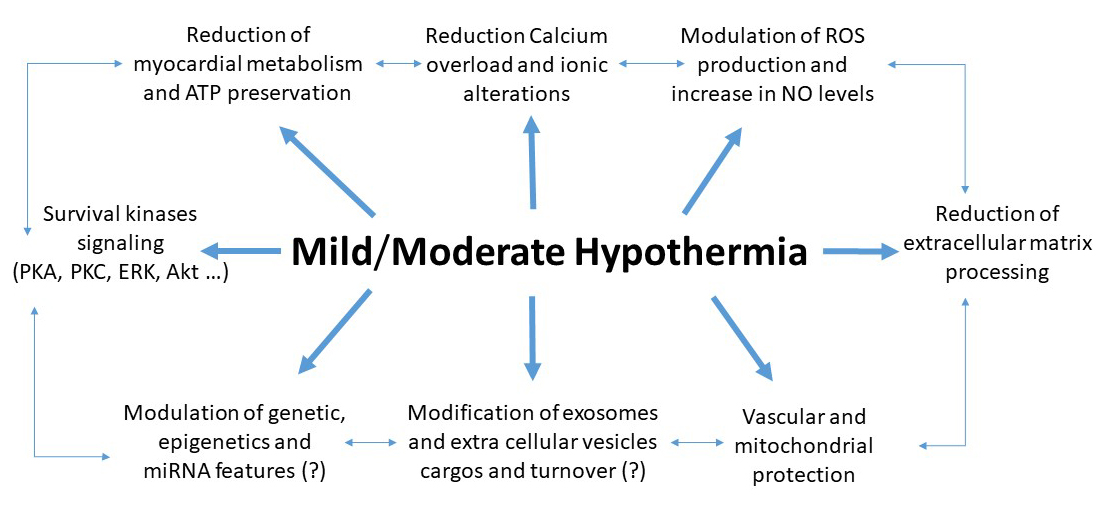
In a new window | Download PPT
Figure 1: Main mechanisms of hypothermia induced cardioprotection. It is likely that these mechanisms are interconnected.
3.1 Effects of hypothermia on heart metabolism
Hypothermia protects ischemic myocardium by reducing the accumulation of dangerous metabolites and ATP usage. In addition, hypothermia also downregulates ATP production by mitochondria. Therefore, energy supply/demand ratio alterations may occur under hypothermic conditions.
Mild/moderate hypothermia (below 31 °C) has been reported to reduce myocardial injury caused by simulated ischemia/reperfusion (I/R) protocols by activating cardiac signaling pathways for survival. Under hypothermic conditions, energy metabolism, mitochondrial calcium homeostasis, and membrane integrity are saved and can play a key role in cardiac resistance to hypoxia (Huang et al., 2015).
It is said that for every 1 °C by which the body is cooled, cellular metabolism is slowed down by approximately 7% (Erecinska et al, 2003). Studies suggested that a temperature threshold exists and a marked decrease in energy supply/demand occurs around a certain temperature: in a study conducted in isolated rabbit hearts, it has been suggested that the threshold is around 30 °C and that the threshold can be modified by providing glucose as a substrate in a cardioplegic solution. In this study myocardial ATP concentration was lower in 34 °C ischemic hearts than those kept at lower temperature and a close relationship between myocardial ATP concentration and functional recovery was described. Functional recovery was improved by glucose in the group kept at 34 °C (Ning et al., 1998a).
A few studies analyzed whether left ventricular hypertrophy (LVH) may affect myocardial protection by hypothermia or cardioplegic solutions and the possible role of metabolism. For instance, it has been reported that a 2-h mild hypothermia cannot improve long-term survival for hypertensive animals with LVH, despite the reduction in circulating levels of damage markers (cardiac troponin T and N-BNP) observed after treatment with hypothermia (Wang et al., 2016). Yet, cardioplegia also adequately protects the hypertrophic myocardium if the regional myocardial temperature does not rise above 15 °C during cardiac arrest (deep hypothermia). However, hearts with a higher LVH displayed reduced myocardial ATP content at the end of the period of ischemia (Scheld et al., 1985).
3.2. Role of cytosolic calcium on hypothermia protection
Cytosolic calcium plays a major role in IRI, but magnitude and time course of I/R-induced calcium overload remain elusive. As recently reviewed, deep hypothermia alleviates virtually all known deleterious features of ischemia, including calcium overload (Hale and Kloner, 2011; Tissier et al., 2012). The mechanisms of mild hypothermia in beating hearts have been less studied. An earlier study suggested that the anti-ischemic and negative inotropic effects of hypothermia are somehow similar to those induced by calcium antagonists rendering redundant the use of these drugs under conditions of hypothermia (Hearse et al., 1984).
Differences exist between hypothermic ischemia and cardioplegic arrest in terms of calcium handling. Taking advantage of temperature dependent variations in the dissociation constant of fluorescent probes used to measure cytosolic Ca2+ concentration, it has been suggested that cytosolic Ca2+ concentration accumulation occurs during hypothermic ischemia with cardioplegic arrest, as well as during unprotected ischemia, without further net Ca2+ influx upon reperfusion only under hypothermic conditions. Thus, reducing calcium overload in reperfusion. Moreover, mild hypothermia inhibited calcium-induced mitochondrial permeability transition pore (mPTP) opening in ischemic rabbit hearts (Tissier et al., 2009). These findings may have important implications for timing of protective strategies during myocardial ischemia/reperfusion (Stamm et al., 2003). Intriguingly, cold cardioplegia not only blunts mitochondrial calcium overload during ischemia/reperfusion, but also the generation of reactive oxygen species (ROS) at reperfusion (see below) (Riess et al., 2004; Gambert et al., 2004). This ROS reduction may be due to a limitation of the so-called ROS-induced ROS release phenomenon, supported by calcium overload and mPTP opening during reperfusion (Penna et al., 2013; Tullio et al., 2013).
3.3 Temperature and oxidation/nitrosation
Low temperature alters mitochondrial membrane composition and mitochondrial ROS production in muscle cells (Guderley 2004). Low temperatures sustained for 14 days affects free radical lipid oxidation and antioxidant levels in the tissues of different organs (liver, kidneys, lungs, and heart). It has been suggested that the activation of antioxidant enzymes as a consequence of the increase in the rate of lipid peroxidation depends on low temperature exposure time (Nikolaev et al., 2018).
Profound (4 °C) hypothermia during the ischemic period results in a reduction of ROS generation and in an improvement of functional recovery in isolated reperfused (30 min, at 37 °C) rat heart (Gambert et al., 2004). A reduction of ROS generation was observed also during short-term 17 °C ischemia (Riess et al., 2004). In a pioneering work, Magni and colleagues reported that after a period of 1-h deep hypothermia, complete functional recovery was obtained upon return to normothermia; however, 3-hours of hypothermia induced irreversible deterioration of the electrical and mechanical heart function, with an increase in lipid peroxidation on rewarming, resulting in a decrease in reduced glutathione levels. Perfusion with a solution containing chelating iron avoided both the increase in lipid peroxidation and the deterioration in heart function (Magni et al., 1994). These data suggest that iron-dependent lipid peroxidation is responsible for time-dependent cold-induced injury.
Mild hypothermia (32 °C) attenuated ROS generation at reoxygenation in a murine cardiomyocyte model. In this model hypothermia was applied 10-min before reoxygenation and maintained for 1-h, after 90-min simulated ischemia (Shao et al., 2010). Moreover, this mild hypothermia-induced cardioprotection was abolished by inhibitors of Akt and nitric oxide (NO) synthase (NOS) but not by a cyclic GMP (cGMP) inhibitor (Shao et al., 2010). Similar results were demonstrated in an isolated rat heart. In particular, hypothermia increased NO synthesis and maintained mitochondrial membrane potential, while enhancing phosphorylation of both Akt and heat shock protein-27 (HSP-27) during ischemia. The lack of a role for cGMP, together with an enhanced production of NO and a reduced amount of ROS suggest a prevalent role of nitrosylation processes rather than the classical cGMP/PKG signaling pathway in the hypothermia induced protection in this model. The protective effects of hypothermia were abolished with either inhibition of NO generation with L-arginine analogues or blockade of phosphoinositide 3-kinase (PI3K) with wortmannin (Mochizuki et al., 2012). These studies suggest a pivotal role for oxidative/nitrosative signaling in hypothermia-induced cardioprotection.
Also high temperature affects mitochondrial respiration and ROS production (Christen et al., 2018). Indeed, in mammals, heart mitochondria are extremely sensitive to increases in temperature (Lemieux et al., 2017). Nevertheless, the relationship between mitochondria, oxidative status, and temperature has yet to be fully characterized.
3.4 Inflammatory signaling modulation by hypothermia
The redox and inflammatory responses are strictly interconnected in several cardiovascular diseases and following IRI (Hernandez-Resendiz et al., 2018; Ong et al., 2018). Therefore, targeting the inflammatory response is among the most promising protective mechanism of hypothermia (Diestel et al., 2008; Yang et al., 2009, 2010). Studies have shown that hypothermia protects against tumor necrosis factor-alpha-induced endothelial inflammatory dysfunction and apoptosis through a MAPK phosphatase-1-dependent mechanism (Yang et al., 2010) and via inhibition of JNK1/2 and ERK 1/2 phosphorylation (Yang et al., 2009). Moreover, hypothermia reversed I/R-induced modulation and expression of several inflammatory factors (such as cleaved caspase-3, PARP, Fas/caspase-8, Bax, and Bcl-2) and reduced NF-κB-dependent pro-inflammatory gene expression (Diestel et al., 2008; Yang et al., 2009). All in all, these studies support a beneficial role for mild hypothermia against the inflammatory response.
3.5 Role of extracellular matrix processing in hypothermia protection
Ischemia/reperfusion damage activates early extracellular matrix processing and, indeed, an increase in cleavage products of matrix plays a role in determining damages after ischemia/reperfusion leading to heart failure. In a recent study this cleavage was observed in normo-thermic ischemia only (Lauten et al., 2014). IRI not only activated extracellular matrix processing, but also enhanced expression of Collagen 18A1/Endostatin in the heart with dissimilar effects of temperature. These data suggest that processing of myocardial extracellular matrix starts early after I/R and depends on temperature levels, which may influence the progression to heart failure (Lauten et al., 2009).
3.6 Genetic and epigenetic effects of mild hypothermia
Besides modulating both myocardial energy requirement and production, myocardial protection induced by hypothermia may be accompanied by an up-regulation of the expression of genes regulating heat-shock-proteins and nuclear-encoded mitochondrial proteins, such as the beta subunit of F1-ATPase (beta F1-ATPase) and the adenine nucleotide translocator isoform 1 (ANT1). Maintenance of gene expression for mitochondrial proteins suggests that signaling for mitochondrial biogenesis or re-synthesis is preserved even after ischemic challenges. Ning et al. (1998b, c) showed that mRNA expression for ANT1 and beta F1-ATPase was maintained in a pattern consistent with the temperature threshold phenomenon (a marked decrease in energy demand occurs at 30 °C, suggesting that this temperature is a sort of threshold), whereas HSP70-1 expression was not influenced by temperature during ischemia (Ning et al., 1998b, c; Xu et al., 1998).
Epigenetics can be defined as meiotic and mitotic changes in gene expression that do not involve a change in DNA sequence and can be heritable. Two major areas of epigenetics, DNA methylation and histone modifications, may have profound effects on controlling gene expression. A few studies have analyzed the effect of temperature on epigenetics. Among these, studies in fishes, flies, mosquitos, and plants have reported temperature-induced epigenetic inheritance (Bonasio 2015; Xu et al., 2019). A study on male mice exposed to cold induced alterations in DNA methylation in their sperm associated with alterations in gene expression that resulted in metabolic changes in offspring (Sun et al., 2018). To the best of our knowledge, the only study that examined epigenetics during therapeutic hypothermia in cardiac pathophysiology is the study by Liu et al. (2019). In this study cardiac proteomes revealed that cold-inducible RNA binding protein (CIRBP) was decreased in rats exposed to chronic hypoxia during cardiopulmonary bypass. Methylation analysis of neonatal rat cardiomyocytes and myocardium samples from patients with chronic hypoxia showed that hypoxia induced hypermethylation of the Cirbp promoter region, resulting in its downregulation and failure to respond to cold-stress. While Cirbp-transgenic rats displayed enhanced hypothermic cardioprotection, cirbp-knockout rats displayed an attenuated response. Analysis of epigenetic modification of Cirbp might help to personalize the use of therapeutic hypothermia in cardiac pathophysiology.
3.6.1 miRNA and Hypothermia
In a variety of conditions, including organ injury and temperature variations, organs and cells release tissue-specific circulating micro ribonucleic acids (miRNAs) into the blood. miRNAs were first described three decades ago as small non-protein coding RNAs (Lee et al., 1993). They bind to messenger RNA to regulate messenger RNA expression. By modulating cellular proliferation, differentiation, and programmed cell death, microRNAs may have a pivotal role in many clinical conditions, including myocardial infarct, tumorigenesis, and metastases. Circulating miRNAs, released from injured myocardium could have some prognostic value in patients who have suffered acute coronary syndromes (Widera et al., 2011).
In a recent article Squiers et al. (2015) wondered whether miRNA modulation might affect an old strategy such as hypothermic organ protection. In an ischemic porcine cardiogenic shock model, the release pattern and plasma level of cardiac-specific miR-208b and liver-specific miR-122 during therapeutic mild hypothermia (33 °C) have been examined. The results of this study indicated that therapeutic hypothermia blunted the increase in miR-122 only. Data from this study confirmed that liver-specific miR-122 is released into the circulation in the setting of cardiogenic shock and suggested that therapeutic hypothermia reduces the plasma levels of miR-122. Yet, treatment with hypothermia after cardiac reperfusion did not modify the plasma levels of miR-208b (Andersson et al., 2012).
Recently, Wang et al. (2015) reported that inhibiting miR-29c with antagomiR-29c (an antagonist of miR-29c) protected the rat brain in a model of prolonged hypothermic circulatory arrest. Notably, miRNA miR-29c is a regulator of peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α), which is a modulator of mitochondrial biogenesis and regulator of mitochondrial metabolism. Therefore, this study confirms and improves our knowledge on the effects of hypothermia in modulating metabolism. In a study aimed at testing small non-coding RNAs as references in quantitative RT-PCR expression analyses, it has been found that RNU6B is a useful marker for differentiating hypothermia deaths from chronic ischemic heart disease deaths (Kaija et al., 2020). Stammet et al. (2012) measured serum miRNA levels 48-h after cardiac arrest in patients that had undergone therapeutic hypothermia. These authors found that miR-122 and miR-21 levels were particularly high in patients who had poor neurologic outcomes. However, neuron-specific enolase (NSE) was a more accurate predictor of neurologic damage than the miRNAs. Yet, these miRNAs did not correlate with markers of myocardial injury or inflammation in these patients. These and other studies (Gilje et al., 2014; Devaux et al., 2015; Lin et al., 2015) pave the way for future studies to confirm the role of miRNAs as new biomarkers of organ damage or protection, and as therapeutic targets during hypothermia and cardiac arrest.
3.7 Effects of temperature on exosomes and extracellular vesicles
Exosomes and extracellular vesicles (EVs) are miRNAs carriers. They carry many other factors and have been implicated in intercellular communication, including transmitting protection or malignancy. It has been demonstrated that adult cardiomyocytes release heat shock protein (HSP-60) both as free protein and carried in exosomes. Whether hypothermia modifies the release of cardiac exosomes and extracellular vesicles is unknown. To the best of our knowledge, while a number of studies considered the role of exosomes and EVs either during fever or hyperthermia (Tytell, 2005), demonstrating that, for example, fever changes exosome permeability (Malik et al., 2013), only a few studies considered exosomes as a biomarker of hypothermia injury/protection. It seems that the turnover of exosomes in the peripheral blood is sufficiently rapid to be clinically useful to evaluate the therapeutic response during hypothermia (Goetzl et al., 2017). This is a fertile field to be studied.
4. Deep/profound hypothermia to improve cardioplegia in mature and immature hearts
Cardioplegia is protective in the ischemic adult heart both under normothermic and hypothermic conditions regardless of whether it is administered once or multiple times. The additive protective effect of hypothermia and chemical cardioplegia in adult dogs has been previously reported (Rosenfeldt et al., 1980). Nowadays, the standard technique of heart preservation is cardiac arrest followed by static-cold-storage (SCS) in a crystalloid preservation solution. These procedures have been reviewed several times. Here we mention only the key steps.
Several strategies have been considered to improve heart storage. These include, supplementation of SCS with hyperpolarizing and/or anti-ischemic agents, novel formulations of heart preservation solutions, donor heart perfusion with perfusion systems, perfusion (persufflation) of donor heart with gaseous O2, pre- and post-conditioning of cardiac graft (see below), and/or donor heart preservation at sub-zero temperature (for review see Minasian et al., 2015).
Several studies evaluated the influence of temperature to preserve donor heart and to minimize cardiac dysfunction caused by IRI, which would inevitably occur during the ex vivo transport period without protection. Although myocardial O2 consumption decreases drastically during SCS of the donor heart at +4 °C, several attempts to further minimize cardiac energy demand and to better preserve heart function by myocardial cooling to different or sub-zero temperatures have been attempted. For instance, Natsuaki et al. (1983) evaluated the optimal myocardial temperature during 4-h ischemia induced by blood potassium cardioplegia. They compared moderate hypothermia and deep hypothermia and two perfusates (with blood or without blood). Indeed, in blood potassium cardioplegia (GIK cardioplegia) at moderate hypothermia, dangerous acid metabolites were scarcely produced and cardiac function was recovered. Specifically, the authors observed a decrease in pH to 6.88 of coronary sinus blood during unprotected reperfusion and to pH 7.22 after GIK cardioplegia, suggesting better preservation of high energy phosphate (Natsuaki et al., 1983). These authors concluded that blood GIK cardioplegia at moderate hypothermia allows the heart to tolerate 4-h ischemia without an excessively elevated coronary perfusion pressure during cardioplegic infusion. Subsequently Sakaguchi et al. (1996) evaluated the ability of the hypothermic simple immersion technique to individuate the temperature providing the best protection against prolonged ischemia. These authors concluded that hearts subjected to subzero temperatures resulted in significantly better cardiac output, aortic flow, and aortic systolic pressure than the control group. Myocardial ATP, ADP, and total adenine nucleotides at end-storage were better preserved. The authors proposed that storage in the intracellular type solution at subzero nonfreezing (-1 °C) temperatures as compared with 4 °C appears to prolong myocardial preservation improving the enhancement of post-ischemic functional recovery, preservation of myocardial adenine nucleotides during ischemia, and prevention of myocardial edema at reperfusion. To prevent irreversible cell injury induced by freezing, cardioplegic solutions have been enriched with anti-freeze proteins (AFPs) endowed with cryoprotective properties. For example, rat hearts stored in the University of Wisconsin (UW) solution containing AFP-I or AFP-III at −1.3 °C for 21-h showed better left ventricle function and a lower occurrence of apoptosis after heterotopic transplantation when compared with controls (Amir et al., 2004). A promising method of sub-zero heart storage has been proposed: in this method, rat hearts were placed in a UW-solution and submerged into a variable magnetic field at minus 3 °C. The hearts were stored for 24-h and then subjected to 120-min reperfusion. The sub-zero storage resulted in decreased tissue edema and an amelioration of post-ischemic left ventricle function and myocardial ATP level (Kato et al., 2012). However, Bernard et al. (1998) using P-31 magnetic resonance spectroscopy and biochemical analyses compared the influence of two different cold temperatures (below 10 °C) for cardiac ischemia by measuring a large variety of metabolic and hemodynamic parameters during ischemia and reperfusion. They concluded that storage at +7.5 °C provides better protection than storage at +4 °C.
The picture is further complicated if immature hearts (heart of a few days old animal) are considered. Indeed, detrimental or beneficial effects of hypothermic cardioplegia in the immature hearts have been reported. For instance, studies have demonstrated that single-dose cardioplegia is protective under different hypothermic conditions in the immature heart. However, multidose cardioplegia affords even greater protection at 20 °C but was deleterious at 10 °C. These studies concluded that, in contrast to the situation in the adult, more "extreme" hypothermia may be damaging in the immature heart when combined with multidose cardioplegia (Kempsford and Hearse, 1990). The same group of authors confirmed that in the neonatal rabbit heart the cardioprotective properties of multidose cardioplegia relative to single-dose cardioplegia are progressively lost as the heart temperature decreases (Murashita and Hearse, 1991). Therefore, the ideal temperature for heart storage is still a matter of debate, and may be influenced by several factors, including the age of the heart donor. The reader is redirected to specific reviews on this topic for a more in depth discussion (e.g. Minasian et al., 2015; Macdonald et al., 2016).
5. Possible adverse effects of hypothermia
Recently, two systematic reviews on adverse effects following therapeutic hypothermia have been published (Karcioglu et al., 2018; Lindsay et al., 2018). From these two reviews one can understand that several adverse effects and/or complications can occur during hypothermia in patients resuscitated from out-of-hospital cardiac arrest. The lower the temperature is, the higher the risk of complications (temperature below 30 °C jeopardize life). Side effects include important cardiac adverse effects such as ventricular dysrhythmias and coronary vasoconstriction, as well as coagulopathies. Regarding this latter side effect, it has been suggested that hypothermia progressively impairs platelet aggregability and coagulation cascade, thus impairing clot formation and hemostasis (De Robertis et al., 2015). Mild hypothermia (33-37 °C) slightly reduces platelet adhesion only, while temperatures below 33 °C reduced both platelet function and coagulation cascade efficiency, resulting in hemostasis defects (Wolberg et al., 2004). Contradictory results have been reported about platelet aggregatory response to classical aggregatory stimuli in the presence of mild/moderate hypothermia (Scharbert et al., 2006, 2010). Therefore, whether platelet dysfunction is a central mechanism of hemostasis impairment during mild/moderate hypothermic strategies remains to be ascertained by further studies.
As said, mild/moderate therapeutic hypothermia has been extensively used to protect the heart and the brain in several clinical settings. In many of these studies the risk of severe adverse effects associated with therapeutic hypothermia seems to be very low (Polderman, 2009; Nielsen et al., 2011; Delhaye et al., 2012). It is likely that a controlled process of cooling in hospital does not induce the side effects observed in accidental hypothermia or in pre-hospital therapeutic hypothermia. Indeed, the aforementioned systematic reviews conclude that therapeutic hypothermia does not cause unfavorable effects leading to significant alterations in the outcomes of the patients following resuscitation (Karcioglu et al., 2018; Lindsay et al., 2018). Also, from the clinical studies reported in Table 1, it can be deduced that the safety of these strategies is acceptable. However, the possibility of major side effects should always be considered during therapeutic hypothermia.
6. Temperature and conditioning
Increased myocardial tolerance to IRI after brief (a few minutes) episodes of coronary ischemia–reperfusion is referred to as ischemic preconditioning (PreCon) (Murry et al., 1986). Ischemic PreCon triggers the most effective endogenous cardioprotective mechanisms developed thus far (Penna et al., 2015). Distinct windows of opportunity for clinical use of conditioning procedures have been elucidated: whilst the conditioning stimulus is applied before the index ischemia in PreCon, it is applied immediately after the index ischemia in postconditioning (PostCon). PreCon and PostCon ischemic stimuli (a few minutes of intermittent ischemia) can be applied in a different/remote organ, which are referred to remote PreCon and remote PostCon, respectively. In the case of ischemic remote conditioning, the procedure of intermittent ischemia can also be performed during the index event, in the so-called remote per-conditioning (Per-Con) (Penna et al., 2015) (Fig 2).
As described above, traditionally, hypothermia is used prior to surgery as a protective measure under conditions of prolonged organ ischemia, such as organ transplantation and open-heart surgery. The therapeutic efficacy of hypothermia in acute cases of ongoing ischemia such as myocardial infarction, stroke, and cardiac arrest has been considered. Here we consider and discuss the possibility of inducing protection in the three distinct windows (PreCon, Per-Con, and PostCon) with temperature variations alone or in association with ischemic conditioning (Fig 2), especially in acute settings.
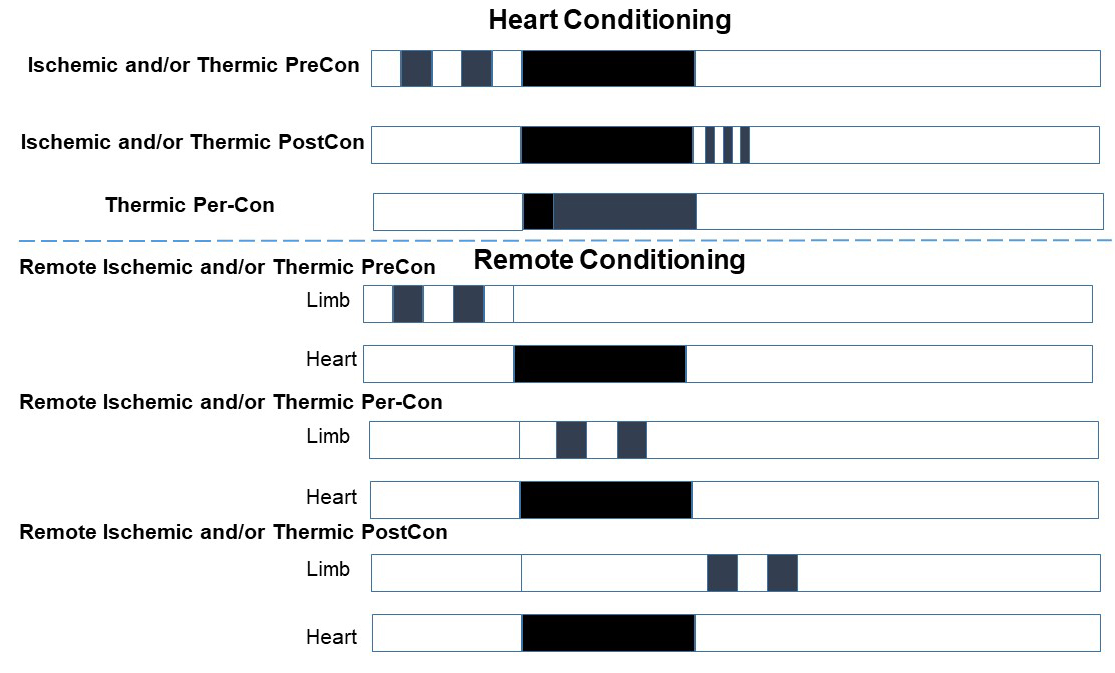
In a new window | Download PPT
Figure 2: Schematic representation of the various protocols of ischemic or thermic conditioning. White boxes represent time of normal perfusion or reperfusion; black boxes are periods of index ischemia; dark gray boxes represent periods of conditioning with brief ischemia or thermic procedures. The number and duration of conditioning cycles used can vary with different experiments and clinical conditions.
PreCon: preconditioning; PostCon: postconditioning; Per-Con: perconditioning.
6.1 Preconditioning procedures
PreCon with ischemia and/or temperature variations can be studied in animal experiments in the setting of myocardial infarction. For successful translation of myocardial PreCon to clinical use at least two conditions are necessary: 1) the predictability of ischemia onset and 2) direct access to the heart; both of these conditions are fulfilled during programmed revascularization interventions and during heart transplantation. We review first the studies considering temperature variations and ischemic PreCon for heart storage, then the studies analyzing these two procedures alone or in combination in the setting of acute ischemia/reperfusion in the beating heart.
6.1.1 Ischemic PreCon plus hypothermic heart storage
It seems that when used in association with the standard techniques of heart preservation, ischemic PreCon may be of value in protecting the heart against IRI. Karck et al. (1996) were among the first to demonstrate that ischemic PreCon enhances donor heart preservation in the setting of prolonged heart storage with hypothermia. They reported an improved post-ischemic left ventricle performance recovery after a single 5-min cycle of normo-thermic I/R just prior to 10-h of heart storage at 4 °C. Also Landymore et al. (1998)reported that one episode of ischemic PreCon can reduce myocardial stunning and can increase tissue ATP levels after heart transplantation. Moreover, it has been reported that the protective effect of ischemic PreCon on cardiac grafts could be further augmented by pharmacological preconditioning with the inhibitor of the Na+/H+ exchanger, cariporide (Kevelaitis et al., 2001).
Zhu et al., (2004) demonstrated that ischemic PreCon obtained with brief intermittent ischemia of a certain duration (between 5- and 15-min) could attenuate the IRI also on immature rabbit hearts when St. Thomas crystalloid cardioplegic solution is applied during sustained mild hypothermic (32 ºC) ischemia period. Therefore, it seems that in this delicate setting of immature hearts, ischemic PreCon can allow the use of less dangerous hypothermic approaches. Despite these positive experimental results, to the best of our knowledge the combination of donor heart hypothermic storage and ischemic PreCon has not been performed in clinical scenarios.
6.1.2 Temperature PreCon and/or ischemic PreCon in the beating heart
Halestrap and collaborators reported the possibility of preconditioning the heart with intermittent hypothermia in a similar fashion to ischemic PreCon (Khaliulin et al., 2007, 2010, 2011). They observed that brief episodes of moderate hypothermic perfusion (< 32 °C), separated by brief periods of normothermic (37 °C) myocardial perfusion, are cardioprotective against infarct size induced by normothermic infarcting ischemia and 60-min reperfusion (37 °C). This procedure is referred to as cold temperature preconditioning (cTP).
These authors demonstrated that cTP could protect hearts against IRI better than ischemic PreCon (Khaliulin et al., 2007). They showed that cTP is more protective than a single 6-min hypothermic perfusion at conferring cardioprotection. Either ischemic PreCon or cTP protocols augmented myocardial levels of high energy phosphates. Moreover, cTP reduced necrotic injury, improved hemodynamic recovery, and decreased arrhythmias during reperfusion. Assessment of myocardial NAD+ levels and calcium-induced swelling of mitochondria isolated after 3 min reperfusion suggested greater inhibition of the mPTP during reperfusion by cTP than ischemic PreCon; these effects were associated with decreased protein carbonylation (marker of oxidative stress). Therefore, the protective effects of cTP may be due to inhibition of mPTP formation and redox stress. Indeed, cTP enhanced protein kinase C-epsilon (PKCƐ) translocation to the particulate fraction and pre-treatment with a PKC inhibitor blocked the cardioprotective effect of cTP. The phosphorylation of AMP-activated protein kinase (AMPK) was also upregulated by cTP. Thus, cardioprotection by cTP was partially blocked by an AMPK inhibitor. The presence of N-(2-mercaptopropionyl) glycine during cTP also abrogated the protection, indicating an involvement of ROS in the cardioprotective signaling mechanism. Therefore, the protective signaling pathway may initially involve ROS signaling with subsequent activation of PKCε and AMPK, thus resulting in reduced opening of the mPTP. Subsequently Halestrap and colleagues, using a pharmacological approach, confirmed that the signal transduction pathway of cTP involves PKC activation that follows protein kinase A (PKA) activation (Khaliulin et al., 2010). It has been also proposed that hypothermia applied before ischemia saves ATP stores during subsequent ischemia/reperfusion and preserves myocardial function at reperfusion. In this study enhanced signaling for mitochondrial biogenesis is also present during a 12-fold increase in HSP70-1 mRNA induced by hypothermia, as shown by beta-F1-ATPase and ANT1 mRNA levels (Ning et al., 1998a).
Halestrap’s group studied also the optimal temperature for TP and whether cTP further augments protection provided by hypothermic ischemia with or without cardioplegia. cTP at 26 °C resulted in the strongest cardioprotection, augmented the concentration of cAMP, and the activity of PKA. On the contrary, cTP at 7 °C aggravated IRI, and had no effect on either cAMP concentration or PKA activity. The authors concluded that cTP at 26 °C bestows additional protection to hypothermia and cardioplegia that could be used during heart surgery and transplantation (Khaliulin et al., 2011).
Studies examined whether combining ischemic PreCon and regional hypothermia during coronary artery occlusion could provide better cardiac protection than either intervention alone. These studies obtained conflicting results. A study by Hale and Kloner (1999) showed that the combination therapy significantly improved regional myocardial blood flow in the previously ischemic region to a greater extent than either treatment alone (Hale and Kloner, 1999). In a study by van den Doel et al. (1998) moderate hypothermia (30-31 °C) modestly reduced infarct size, but this limitation was attributed to a reduction in the at risk area. However, moderate hypothermia markedly enhanced the cardioprotection induced by ischemic PreCon in the beating rat heart. Therefore, the authors concluded that the combination could limit the irreversible damage produced by longer periods of coronary artery occlusions (van den Doel et al., 1998). In a study by Juggi et al. (1997), ischemic PreCon when combined with hypothermia attained no additional protection. In another study moderate/deep hypothermia (23 °C) did not affect the protective effects of ischemic PreCon, but ischemic PreCon was no longer protective in profound hypothermia (6-8 °C) (Takeshima et al., 1999).
Miki et al. (1998) tested whether cooling established before or during ischemia can limit infarction and be additive to protection observed with ischemic PreCon. In this study rewarming started upon reperfusion in all protocols. The authors demonstrated strong cardioprotective effects of moderate cooling (32 °C) started before ischemia and a less potent protection when cooling was instituted 10- or 20-min after the onset of ischemia. Nevertheless, only this latter protection (hypothermic Per-Con; see below) could augment the protective effect induced by ischemic PreCon. The authors suggest that the cooling and ischemic PreCon use different mechanisms. If confirmed this is an important observation as cooling after the onset of ischemia can be considered a sort of Per-Con, which has the potential to be used in the clinical arena during myocardial infarction. Actually, a study suggests that ischemic PreCon induces additive protection with mild hypothermic myocardial ischemia by improving mitochondrial bioenergetics during and after ischemia. In this study a variation in NADH and flavin adenine dinucleotide (FAD) levels were considered a sign of improved mitochondrial redox balance during and after mild hypothermic ischemia and were attributed to mitochondrial KATP channel opening (An et al., 2005). Therefore, it seems that cooling can be easily induced and is well tolerated by the heart. It is not harmful and in some conditions hypothermia can protect the heart against infarction especially when it is started immediately after the onset of coronary occlusion. Moreover, in programmed events hypothermia can be additive with protection induced by ischemic PreCon.
Yet, we should consider that ischemic conditioning during simultaneous hypothermia may not be effective because hypothermia could not only be protective against ischemia, but it could also prevent ischemia from triggering cardioprotective pathways. This is supported by studies examining the efficacy of ischemic PreCon at different temperatures (Dote et al., 1998; Ghadhanfar and Juggi, 2007). Ghadhanfar and Juggi compared the efficacy of ischemic PreCon induced in hypothermic (10, 20 or 30 °C), normothermic (34 or 37 °C), or hyperthermic (40 degrees C or 42 °C) conditions, in the isolated rat heart model. In this study PreCon was induced by two episodes of 5-min ischemia at the designated PreCon temperature and 10-min of reperfusion, respectively. Subsequently, hearts were subjected to global ischemia at 34 °C for 60-min followed by reperfusion for 30-min at the same temperature, during which the post ischemic recovery in contractile and coronary vascular functions was assessed. Hyperthermic ischemic PreCon provided optimal cardioprotection and increased tolerance to ischemia. Hypothermia plus ischemic PreCon was comparatively less effective, and normothermia plus ischemic PreCon resulted in an intermediate increase in tolerance to ischemia. These data support the idea that hypothermia increases the threshold for ischemic preconditioning. An idea that was previously suggested by other authors (Lu et al., 1997; Dote et al., 1998). The results of these studies indicate that the extent of reversibility of ischemic damage depends on the preconditioning temperature. Therefore, it is suggested that hypothermia (<34 °C) during the preconditioning period must be avoided to elicit the best protection against infarction by ischemic PreCon, and that the ideal ischemic PreCon temperature is higher (between 40 and 42 °C) than normothermia (37-38 °C).
6.1.2.1 Protection by hyperthermia
Actually, hyperthermia was the first stress shown to trigger the synthesis of heat stress proteins (HSPs). In particular, a study in 1983 (before ischemic preconditioning was introduced) reported that whole body hyperthermia (42 °C for 15 min) could induce the expression of HSP70 in the heart and other tissues (Currie and White, 1983). Subsequently it was reported, by the same group, that pre-ischemic hyperthermia could improve post-ischemic cardiac functional recovery (Currie et al., 1988). Since then several studies have reported that the heart can be preconditioned by heat stress (HS). It seems that HS-PreCon is able to induce only delayed myocardial protection against IRI, which is similar to the second window of protection induced by ischemic PreCon (Pagliaro et al., 2001). HS-PreCon can reduce cellular necrosis, preserve ventricular and coronary endothelial functions, and limit arrhythmia occurrence (reviewed in Joyeux-Faure et al., 2003). A recent study confirmed that a long period of recovery (72-h) between HS-Precon and achievement of protection is necessary (Ilievska et al., 2018). The main reason for this delayed protection is because the mechanisms that underlie HS-PreCon mainly involve new synthesis of proteins that require time, such as HSPs and antioxidant enzymes induced by heat and by redox signaling. Indeed, several studies have shown that HS-PreCon induced by whole body hyperthermia (40-42 ° C for 15-20 min) is associated with induction of HSPs (Yamashita et al., 1998; Kevelaitis et al., 2001; Xi et al., 2001; Broderick, 2006; Vogt et al., 2007), but some studies questioned whether these proteins or antioxidant enzymes are the principal end-effector of the protection (Abe et al., 2000; Xi et al., 2001). It seems that whole body hyperthermia can induce delayed protection against various forms of injury in heart, brain, liver, lungs, skeletal muscle, and the small intestine, as well as against lethal endo-toxemia. An increase of body temperature to 39-42 ° C has been also suggested as novel treatment against the increase in oxidative stress in obesity-related metabolic syndromes and diabetes (Medina-Navarro et al., 2009; Okada et al., 2004). In cardiac protection against IRI a role for PKC, MAP kinases, ATP-sensitive potassium channels, ROS, and NO in the protective signaling cascade has been reported (Yamashita et al., 1998; Kevelaitis et al., 2001; Xi et al., 2001; Joyeux-Faure et al., 2003; Broderick, 2006; Vogt et al., 2007).
Whole body hyperthermia does not specifically target the organ of interest and may have adverse side effects on blood, potentially limiting the safety of HS-PreCon (Walker et al., 1993). Therefore, some studies have analyzed the possibility of inducing cardioprotection using a lower temperature for longer periods or heating only the heart with some device. Su et al. (1999) used a 39 °C pre-conditioning, which triggered thermo-tolerance and oxidative resistance in H9c2 cardiac myoblasts. Unfortunately, this study was conducted only in vitro and deserves validation in other models. A more interesting approach is that proposed by Motamedi and coworkers, who used heating probes that required direct mechanical contact with the surface of the heart or a radiant volumetric laser that did not require a direct contact for sub-lethal local heating of the heart (42 °C for 20 min). Both approaches improved myocardial salvage by preconditioning the myocardium against reperfusion injury 4 hours after the heating application (Gowda et al., 1998; Gong et al., 2008). Unfortunately, to elevate and maintain tissue temperature at the desired temperature with these devices the chest must be opened. To the best of our knowledge no studies have attempted “local heating” with an intravascular or transthoracic device, which would be more appealing for clinical use.
6.1.3 Per-Conditioning and Postconditioning procedures
Ischemic PostCon is the intriguing phenomenon whereby brief stimuli (e.g. a few seconds of intermittent ischemia) are applied in one organ immediately after artery reopening to render the tissue resistant to reperfusion injury. Per-Con can be attained by applying the stimuli (e.g. brief remote ischemia and/or organ cooling) during the index ischemia. The paradigms of ischemic Per-Con and PostCon may be particularly relevant, as they extend the usefulness of conditioning procedures to reduce reperfusion injury into the clinical arena.
The application of hypothermia during ischemia may be considered a sort of hypothermia Per-Con. The majority of authors agree on the effectiveness of cooling during ischemia starting as early as possible (hypothermia Per-Con: reducing the temperature earlier by 3 °C may reduce infarct size similarly to ischemic PreCon); however a debate exists regarding the benefit of mild hypothermia applied during reperfusion (hypothermia PostCon) to limit IRI (Tissier et al., 2011). The majority of studies suggest that hypothermia limits ischemic injury rather than reperfusion injury (Hale et al., 1997; 2003; van den Doel et al., 1998; Tissier et al., 2007,2009).
As mentioned above, in a study where moderate cooling was applied immediately after the onset of acute myocardial ischemia (i.e. hypothermic Per-Con) Miki et al. (1998) observed an appreciable reduction in infarct size. More recently it has been confirmed that mild hypothermia can ameliorate heart recovery when applied during ischemia (Stadelmann et al., 2013) or during ischemia and reperfusion (Shao et al., 2007, 2010; Hamamoto et al., 2009).
The beneficial effects of hypothermia during ischemia (hypothermia Per-Con) on cardiomyocyte function, infarct size, no-reflow phenomenon, and cardiac remodeling have been demonstrated several times in different animal models (Hale et al., 2011; Dai et al., 2015). Energy preservation and intracellular signaling pathways, including PKC, Akt/PKB, ERK, PI3K, endothelial NOS and NO, have been reported to mediate cardioprotection afforded by mild hypothermia Per-Con (Yang et al., 2011; Tissier et al., 2012; Mochizuki et al., 2012; Shao et al., 2010).
To the best of our knowledge a few studies demonstrated a positive role for temperature variation in PostCon procedures. In particular, Götber et al. (2011) reported an 18% reduction in infarct size when hypothermia started 40 minutes after the onset of ischemia and 5 minutes before the reperfusion (the total duration of ischemia was 45 min). In this study, a greater reduction in the size of the heart attack was obtained when hypothermia was started 25 minutes before the reperfusion and was maintained for 20 minutes during the reperfusion. However, the latter procedure does not exclude the possibility that the protection was due to hypothermic Per-Con rather than hypothermic PostCon. In a study in which mild hypothermia (30 °C) was instituted at the beginning of reperfusion for a brief period (10-min), improvements in post-ischemic developed left ventricular pressure, heart rate, contractility, cardiac output, and cardiac work, were observed compared with controls. These effects were accompanied by a reduction in necrosis (reduced LDH release), improved endothelial function (higher coronary flow), increased oxygen consumption, and a trend toward less cytochrome c release; thus suggesting better metabolic recovery (Farine et al., 2016). Moderate cooling also resulted in protection when applied during reperfusion for 60-min (Tolboom et al., 2016) or 180-min (Mochizuki et al., 2012). Recently, acceleration of autophagic flow and mitophagy has been reported as a central mechanism in the recovery of cardiomyocytes and mitigation of post-infarct remodeling when hypothermic conditions were induced 30-min after the beginning of reperfusion (Marek-Iannucci et al., 2019). Therefore, it also seems that reperfusion injury, in some circumstances, can be reduced with hypothermia. Moreover, the beneficial effect of ischemic PostCon could be additive with the beneficial effects of hypothermic Per-Con. For instance, an ischemic PostCon protocol consisting of three 30-s episodes of global ischemia performed after 4-h of hypothermic rat heart storage (4-h of cold ischemia and cardioplegic protocol) resulted in improved post-ischemic LV function, reduced superoxide anion production, and suppressed reperfusion arrhythmias (Lauzier et al., 2007). In addition, hyperthermia-induced cardioprotection was potentiated by ischemic PostCon (Murozono et al., 2009). In this study hyperthermia (43 °C for 20 min) was applied 24-h before ischemia onset. Ischemic PostCon obtained with 5 cycles of 10-sec reperfusion/10-sec ischemia potentiated the cardioprotective effects of hyperthermia likely via enhancement of reperfusion-induced Akt phosphorylation. Nevertheless, the opening of the mitochondrial ATP-sensitive potassium (mitoKATP) channel, which is involved in hyperthermia-induced cardioprotection, is not necessary to potentiate protection induced by ischemic PostCon. However, these PostC studies need to be further confirmed in other models as a number of authors are convinced that thermic approaches cannot reduce reperfusion injury (Tissier et al., 2012).
6.2 Remote ischemic conditioning procedures and temperature
Remote ischemic conditioning (RIC) is the interesting phenomenon whereby brief, non-lethal episodes of ischemia and reperfusion applied in one organ, tissue, or vascular bed renders the whole body and each organ resistant to IRI (Candilio et al., 2013) (Fig 2). RIC can be attained in animals with brief episodes of ischemia/reperfusion in mesentery, kidney, or limbs. Besides remote ischemic PreCon, in which brief intermittent periods of ischemia (generally 3-4 cycles of 5 min ischemia) are applied before the onset of index myocardial ischemia, cardioprotection can also be attained with concurrent application of the remote ischemic stimulus during index coronary occlusion (remote ischemic Per-Con), or at the time of reperfusion (remote ischemic PostCon) (Przyklenk et al., 2011; Vinten-Johansen and Shi, 2011; Heusch et al., 2015). Studies revealed no apparent difference in efficacy of ischemic remote PreCon, Per-Con, and remote PostCon (Basalay et al., 2012; Zhu et al., 2013; Penna et al., 2015). Limb RIC significantly reduced infarct size in humans and in animal in vivo models of myocardial IRI. However, the recent large multicenter 5401 ST-elevation myocardial infarction (STEMI) patients CONDI2/-ERIC-PPCI trial, failed to show any clinical benefit of limb RIC, with no differences with respect to control. Intriguingly, in a systematic review and meta-analysis a significant heterogeneity in effect size was described that could not be explained by any of the experimental variables analyzed by meta-regression (Bromage et al., 2017). From the analysis it emerged that studies lacked consistency in quality and study design. Moreover, to the best of our knowledge no studies considered the temperature of the ischemic limb among the variables to be controlled (limb temperature was also not mentioned in the above meta-analysis). Since in the ischemic limb temperature can decrease in a variable way, it is surprising that this important parameter is not strictly controlled during RIC maneuvers. There is, therefore, an urgent necessity for more well-performed and standardized in vivo studies with particular emphasis on detailed characterization of RIC protocols and investigating the potential impact of limb temperature. Standardized remote conditioning protocol(s) performed at controlled limb temperature may be used to increase the efficacy of this powerful approach. In a preliminary study we have evidence that controlling the temperature of the limbs (avoiding the cooling of the limbs during conditioning) can improve the effectiveness of RIC.
In a recent study it has been proposed that remote ischemic PostCon alone or associated with hypothermia may improve postresuscitation cardiac and neurological outcomes in pigs (Xu et al., 2016). Remote ischemic PostCon was induced in swine by 4 cycles of limb ischemia followed by reperfusion. Hypothermia was achieved by surface-cooling to establish a body temperature of 32-34 °C. In animals that underwent remote ischemic PostCon, a larger stroke volume and an improved cardiac ejection fraction, as well as better cerebral function 72-h after resuscitation, was observed. Also reduced levels of cardiac troponin I and NSE were detected in these animals when compared to the control. The combination of remote ischemic PostCon plus hypothermia resulted in greater recovery in cardiac and neurological function when compared with remote ischemic PostCon alone (Xu et al., 2016). Surprisingly, we cannot find other studies that combined RIC with hypo- or hyperthermia. The possibility that negative results were not published cannot be ruled out.
7. Studies in perspective
Temperature is an important variable affecting IRI that deserve to be strictly controlled and eventually modified to amplify the efficacy of cardioprotective strategies. Nevertheless, more in depth studies are necessary to understand the mechanisms of protection by thermic therapy alone and in association with other cardioprotective interventions. Several issues are still opened. For instance, no studies defined the role of the various cell death types in reducing injury following the application of thermic therapies in the attenuation of IRI, and whether modification of death pathways may be influenced in the presence of co-morbidities (e.g. age, obesity, diabetes, etc). Very few studies analyzed the role of miRNA, exosomes, or microvesicles. These are challenges that must be addressed and overcome for better exploitation of this powerful therapeutic strategy. Since the mechanism of protection by thermic therapy may differ from that of ischemic conditioning (for example, hypothermia during ischemia prevents cardiac stunning, whereas ischemic pre and postconditioning do not), it should be possible to add thermic therapy and conditioning procedures to produce ‘amplified protection’.
8. Conclusions
Besides thermic therapy to better store hearts for transplantation, three distinct time windows are proposed for the use of thermic therapy to reduce IRI in the beating heart. The first window is before ischemia, which is temperature PreCon. This can be applied alone or in association with other strategies in programmed interventions. Cooling or heating during this window may serve to trigger and amplify cardioprotective signaling pathways. The majority of studies support the notion that cooling may blunt ischemic PreCon effectiveness, whereas moderate heating may potentiate it. The second window of opportunity is during ischemia, that is temperature Per-Con. In this window moderate/mild cooling seems to be the only thermic cardioprotective option, while deep cooling may require cardiac arrest. In the beating heart, moderate/mild cooling during this window may target ROS production, calcium, and other ion management, as well as pH control. The third window is the PostCon during early reperfusion (during the first minutes of reperfusion) or during late reperfusion (hours after the beginning of reperfusion), which is termed temperature PostCon. Early thermic PostCon treatment may target mechanisms similar to those of PreCon or Per-Con, whereas late thermic PostCon may target mainly delayed pathways to cell death, and inflammatory and maladaptive processes. Theoretically, an extraordinary opportunity, scarcely explored, is remote temperature conditioning, associated or not with RIC.
It must be recognized, however, that the control of temperature is complex in nature and this complexity may be responsible for the discordant results among various studies in terms of the time course and potency of cooling-induced cardioprotection, especially in humans. These difficulties must not dampen enthusiasm for studying this powerful cardioprotective strategy. Indeed, a better understanding of temperature-triggered myocardial resistance against IRI may have a large spectrum of clinical implications as well as insightful into the thermic biology.
Funding
Claudia Penna, Manuela Aragno, and Pasquale Pagliaro were supported by University of Torino, Italy (PENC_RILO, ARAM_RILO and PAGP_RILO) and by MIUR (PAGP_FFABR_17_01 and by PENC_FFABR_17_01).
References
Pasquale Pagliaro1
1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy.
Manuela Aragno1
1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy.
Claudia Penna1
1Department of Clinical and Biological Sciences, University of Turin, Turin, Italy.
Corresponding author:
Pasquale Pagliaro
Email: pasquale.pagliaro@unito.it
or
Claudia Penna
Email: claudia.penna@unito.it

In a new window | Download PPT
Figure 1: Main mechanisms of hypothermia induced cardioprotection. It is likely that these mechanisms are interconnected.

In a new window | Download PPT
Figure 2: Schematic representation of the various protocols of ischemic or thermic conditioning. White boxes represent time of normal perfusion or reperfusion; black boxes are periods of index ischemia; dark gray boxes represent periods of conditioning with brief ischemia or thermic procedures. The number and duration of conditioning cycles used can vary with different experiments and clinical conditions.
PreCon: preconditioning; PostCon: postconditioning; Per-Con: perconditioning.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 12925 | 42 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA