Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Tilorone, an oral antiviral agent utilized in humans, preconditions against transient ischemia in mice
Time:2020-02-29
Number:11338
Yujia Zhai1,2, Yingxin Chen2, Saravanan S. Karuppagounder2, Amit Kumar2, Nandini Kundu2, Ishraq Alim2, Stephanie Taylor3, Craig E. Brown3, Xiang Luo1, Yi-Fang Li1, Dawei Lian4, Yang Chen4, Hilda Ahnstedt5, Louise D. McCullough5, Rong-Rong He1, Rajiv R. Ratan2
Author Affiliations


- 1Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou 510632, China.
- 2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
- 3Division of Medical Sciences, University of Victoria, Victoria, BC, Canada.
- 4School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou 51006, China.
- 5Department of Neurology, University of Texas Medical School at Houston, USA
Conditioning Medicine 2020. 3(1): 1-8.
Abstract
Tilorone, an antiviral immunomodulator currently used in humans, was previously identified in a cell based screen for activators of hypoxia-inducible factor transcriptional activity. It reduced infarct size by 80% in a rat model of permanent stroke when delivered 24 hours prior to ischemia. Despite these robust preconditioning effects, its efficacy against stroke in mice are unclear and accordingly the use of transgenics to probe its mechanism of action have not been undertaken. Systemic (intraperitoneal; IP) delivery of tilorone (100 mg/kg) reduced infarct size by nearly 70% in a murine model of transient focal ischemia. This protective dose induced alterations in interferon (IFN)-associated mRNAs in the brain and peripheral organs. Forced expression of one of these genes, IFN-induced protein with tetratricopeptide repeats 1 (Ifit1), prevented oxygen-glucose deprivation induced death in hippocampal neuroblasts. In contrast to prior studies, tilorone significantly reduced hypoxia-associated mRNAs including vascular endothelial growth factor (VEGF). Additionally, tilorone was neither effective when delivered intracerebroventricularly, nor did it protect against permanent focal ischemia. Together, these studies suggest that tilorone is a clinically-used inducer of the IFN antiviral response that can precondition mice from deleterious effects of transient ischemic stroke via effects that require a non-CNS site of action. Future studies can now leverage transgenic mice to examine a model where tilorone, a known intercalator, stabilizes DNA to trigger activation of cytosolic DNA sensing and antiviral, homeostatic transcription leading to reduced metabolic demand prior to stroke and subsequent neuroprotection.
Keywords: antiviral, interferons, hypoxia, preconditioning, edema.
Abstract
Tilorone, an antiviral immunomodulator currently used in humans, was previously identified in a cell based screen for activators of hypoxia-inducible factor transcriptional activity. It reduced infarct size by 80% in a rat model of permanent stroke when delivered 24 hours prior to ischemia. Despite these robust preconditioning effects, its efficacy against stroke in mice are unclear and accordingly the use of transgenics to probe its mechanism of action have not been undertaken. Systemic (intraperitoneal; IP) delivery of tilorone (100 mg/kg) reduced infarct size by nearly 70% in a murine model of transient focal ischemia. This protective dose induced alterations in interferon (IFN)-associated mRNAs in the brain and peripheral organs. Forced expression of one of these genes, IFN-induced protein with tetratricopeptide repeats 1 (Ifit1), prevented oxygen-glucose deprivation induced death in hippocampal neuroblasts. In contrast to prior studies, tilorone significantly reduced hypoxia-associated mRNAs including vascular endothelial growth factor (VEGF). Additionally, tilorone was neither effective when delivered intracerebroventricularly, nor did it protect against permanent focal ischemia. Together, these studies suggest that tilorone is a clinically-used inducer of the IFN antiviral response that can precondition mice from deleterious effects of transient ischemic stroke via effects that require a non-CNS site of action. Future studies can now leverage transgenic mice to examine a model where tilorone, a known intercalator, stabilizes DNA to trigger activation of cytosolic DNA sensing and antiviral, homeostatic transcription leading to reduced metabolic demand prior to stroke and subsequent neuroprotection.
Keywords: antiviral, interferons, hypoxia, preconditioning, edema.
1. Introduction
Ischemic preconditioning (IPC) is a time-tested paradigm for delineating resistance to ischemia (Bolli, 1996). IPC is classically induced by short exposures ischemia, and this type of sublethal stress leads to two recognizable phases of neuroprotection against stroke. The initial phase is short lasting involving the activation of pre-existing proteins. The subsequent protective phase is more durable lasting more than 24 hours and involving transcription of a cassette of protective genes (Sassone-Corsi et al., 1989; Stevens et al., 2011). Transcriptional programs that have been implicated in preconditioning involve adaptive responses to bioenergetic stress (Narayanan et al., 2013), viral stress (Stevens et al., 2011; McDonough and Weinstein, 2016), and hypoxic stress (Sharp et al., 2004; Stowe et al., 2011). Indeed, prior studies from numerous labs have shown that small molecule activators of hypoxic adaptation are effective in preconditioning the brain against stroke (Siddiq et al., 2005; Zhou et al., 2017; Karuppagounder et al., 2018). In an attempt to speed evaluation of targets involved in hypoxic adaptation to humans, we screened 2000 compounds, including clinically-approved compounds, using a hippocampal line that stably expressed a hypoxia response element-driven luciferase reporter gene. One drug, tilorone, emerged as far superior to others in activating our reporter (Ratan et al., 2008; Karuppagounder et al., 2018). Tilorone (2,7-bis(2-(diethylamino)ethoxy)fluoren-9-one dihydrochloride; Figure 1A) was the first compound recognized to induce a systemic, antiviral interferon (IFN) response when taken orally (Mayer and Krueger, 1970; Kaufman et al., 1971). While taken off the market in the United States as a possible inducer of mucopolysaccharidoisis, it is still used in other countries under the trade name Amixin or Lavomax to treat emerging viral infection including the common flu, hepatitis, and encephalitis (Sel'kova et al., 2001; Loginova et al., 2004). Its salutary effects result from induction of IFNs, pleiotropic cytokines with established roles in antiviral defense. The ability of tilorone to induce IFNs and robustly precondition the rat brain from ischemic stroke is consistent with prior data that demonstrate that lipopolysaccharide (LPS) and Poly IC can precondition the brain via IFN-dependent mechanisms (Stevens et al., 2011; McDonough et al., 2017). However, tilorone’s ability to robustly activate hypoxia inducible factor (HIF) and its gene products (Ratan et al., 2008), a pathway also implicated in preconditioning, raises the possibility that tilorone possesses the ability to activate multiple protective pathways including hypoxic defense, IFN-mediated antiviral defense, or both. Here we test these possibilities and demonstrate that tilorone’s preconditioning effects observed in rats can be extended to mice; that protection requires a non-CNS site of action; and that protection, while associated with robust induction of the antiviral IFN adaptive response, unexpectedly involves repression of the hypoxic adaptive response in multiple organs including the brain.
Methods
Animals
Experiments on mice or rats were approved by the Weill Cornell Medicine, Jinan University, or University of Victoria Institutional Animal Care and Use Committee and conducted in accordance with the NIH, Canadian Council for Animal Care, or Center for Laboratory Animal care of Jinan University and ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. Eight -12 weeks old male C57BL/6 mice were purchased from Jackson Laboratories and 3 months old CD1 female pregnant mice were purchase from Charles River and housed as previously described (Ratan et al., 1994). Male Sprague-Dawley rats (250-300 g) were purchased from Guangdong Medical Laboratory Animal Center (Guangzhou, China) and housed under clean and ethical conditions.
Murine model of medial cerebral artery occlusion (MCAO)
MCAO surgeries were conducted as described previously (Alim et al., 2019). The individual performing the infarct volume analysis was blinded to treatment group. Mice were euthanized, and brains were collected 24 hours after reperfusion for TTC (2,3,5-triphenyltetrazolium chloride) staining (Sigma, St. Louis, MO).
Rat model of MCAO
Focal cerebral ischemia of MCAO was implemented as previously described (Guan et al., 2012).
Photothrombotic model of stroke in awake mice
The photothrombotic model of stroke was performed as previously described (Seto et al., 2014). Seven days after stroke, mouse brains were removed and prepared for sectioning. Sections were stained for cresyl violet, dehydrated, and coverslipped with Permount. An experimenter blinded to treatment conditions imaged brain sections and then measured the infarct region in every 3rd section (150 µm between each section) using Image J software. Infarct volume was calculated by summing up the infarct area for each section multiplied by the distance between each section.
Magnetic Resonance Imaging (MRI)
MRI was performed on a 7.0-T PharmaScan70/16 MRI scanner (Bruker Bio Spin, USA) by an animal coil. Each MRI session consisted of 25 transverse T2-weighted slices. T2-weighted images were obtained from a 0.8 mm thick coronal section with a 35 mm × 35 mm field of view, TR = 2631 ms, TE = 33 ms, and a matrix of 256 × 256. The lesion volumes were determined by an investigator experienced in experimental stroke MRI with Image J software.
RT2 Profiler PCR array analysis
The treatment mice group received tilorone (100 mg/kg IP) once starting 24 hours before sacrifice and RNA was extracted and quantified as previously described (Karuppagounder et al., 2018). Total RNA (200 ng) was amplified on PCR Array Mouse Type I Interferon Response or Hypoxia signaling PCR Array chips. Raw data were analyzed using the website (www.SABiosciences.com/pcrarraydataanalysis.php). Data analysis was aimed at assessing the effect of drug treatment vs. the vehicle control group.
Cell culture and viability
HT22 murine hippocampal cells and primary cortical neurons were cultured as previously described (Ratan et al., 1994). Cell viability was assessed as previously described (Zille et al., 2017) using an MTT assay and LIVE/DEAD staining.
Mature neuron culture and oxygen-glucose deprivation (OGD) model
E15-17 cortical neurons were plated at 0.25x106 cells/ml density in neurobasal media and maintained for 12-14 days by replacing 50% media by fresh media every 2 days. The mature neurons were treated with different doses of tilorone for 24 hours (preconditioning). Then after washing 3 times with glucose free media, the cells were placed in glucose free media and transferred to a hypoxia chamber under OGD conditions for 3.5 hours. A replicate plate in normal media was incubated under normoxic conditions. The OGD conditions were terminated by replacing the glucose free media with neurobasal media. The cultures were returned to the incubator for 24 hours. MTT and live/dead assays were then performed.
Immunoblot Analysis
Protein extracts were prepared as previously described (Zille et al., 2017). Blots were incubated in antibodies against IFN-induced protein with tetratricopeptide repeats 1 (IFIT1) or occluding, (NOVUS, NBP2-02340) and β-actin overnight at 4°C. Subsequently, blots were incubated in secondary antibodies for 40 min at room temperature. Proteins were detected using Odyssey infrared imaging system (LI-COR Biosciences).
Quantitative real-time PCR
Total RNA was prepared using the NucleoSpin RNA II kit (MACHEREY-NAGEL) according to the manufacturer’s protocol. Duplex real-time PCR reactions were performed with gene expression assays using 6-carboxyfluorescein–labeled probes (Thermo Scientific) for Epo (Mm 00433126_m1), Vegf (Mm 01281449_m1), Ldh (Mm00495282_g1), Ifit1 (Mm00515153_m1), Ifitm1 (Mm00850040_g1), Irf3 (Mm00516779_m1), and Irf5 (Mm00496477_m1). All expression levels were normalized to β-actin gene expression levels, using VIC-labeled probe. All experiments were performed using a 7500 Real-Time PCR System (Applied Biosystems).
Behavioral test
The corner task, tape removal task, pole test ,and Neurological Severity Score were performed as described previously (Karuppagounder et al., 2016; Chen et al., 2001).
Statistical analyses
Data are reported as means ± SEM of multiple individual experiments each carried out in triplicate. Unless stated otherwise, the statistical analyses were carried out with GraphPad Prism 5. A two-tailed t test was used if two groups were compared, a one-way ANOVA with Dunnett’s multiple comparisons post hoc test if more than two groups were compared, and a two-way ANOVA with Bonferroni’s post hoc test if two independent variables were compared.
Results
Preconditioning with the drug tilorone in an MCAO model reduces infarct volumes in rodents
We evaluated the ability of tilorone to precondition mice from brain infarction and behavioral decrement following ischemia. Injection of tilorone (50 or 100 mg/kg IP) 24 hours prior to 45 minutes of MCAO followed by 24 hours of reperfusion (Figure 1A) showed significantly reduced cortical (p < 0.01, 100 mg/kg, data not shown for 50 mg/kg) and total hemispheric (p < 0.01) infarct volumes (Figure 1B) at 24 (mice and rats, Supplementary Figure 1A, B) and 72 hours (rats, Supplementary Figure 1A, B). The effects were dose-dependent and at both doses, tilorone treatment showed improvements in the corner task (a measure of vibrissae, postural, and motor function), the tape removal task (a measure of sensory and motor neglect) and in the pole test (a simple test of motor function) (Figure 1C) as compared to MCAO mice treated with vehicle at 24 hours. Sustained behavioral improvements using a composite neurological score were evaluated only in the 100 mg/kg tilorone group. Significant improvements were seen in the tilorone-treated rats out to 7 days (Supplementary Figure. 1C). Accordingly, from these data, we conclude that pre-treatment with tilorone can successfully precondition against stroke-induced damage and behavioral dysfunction in mice and rats. In order to verify that preconditioning by tilorone occurred distal to a perfusion deficit, we measured cerebral blood flow (CBF) during transient 45 min MCAO (Supplementary Figure 1D). The results showed that during the transient MCAO, CBF reductions were not affected by tilorone preconditioning. Moreover, we found that preconditioning with tilorone (3 µM) protects mature neurons in culture against OGD-induced injury (Figure 1D). These results suggest that not only does tilorone protect in mice, but its efficacy in vitro make unlikely that it needs to be activated by first pass metabolism in the liver.
.png)
In a new window | Download PPT
Figure 1: Pre-treatment with tilorone can precondition against stroke-induced damage and behavioral dysfunction in mice. (A) The chemical structure of tilorone dihydrochloride. Tilorone (Ti) treatment and the MCAO model protocol. (B) Representative images of brain sections after TTC staining 24 hours after transient MCAO. Preconditioning of 100 mg/kg Ti (n = 10 mice/group) significantly reduced infarct size 24 hours after MCAO. Infarct volumes shown are the cortex (p < 0.05 for Ti compared to control) and hemisphere infarct volumes. Data are expressed as mean ± SEM. Students t test was used to calculate the significance. All the treatments were randomized for in vivo and treatment group assignments. (C) Tilorone improved function as measured by the corner task assessed 24 hours after MCAO in both the 50 mg/kg (Ti L) and 100 mg/kg (Ti H) dose groups compared to vehicle group (p < 0.01 for both doses of Ti compared to control). Tilorone also improved the tape removal task, measured 24 hours after MCAO in both the 50 mg/kg (Ti L) and 100 mg/kg (Ti H) dose groups compared to vehicle control (p < 0.05 for Ti H vs. control, p < 0.01 for Ti L vs. control). Tilorone (100 mg/kg) preconditioning improved the total reach time in the pole test measured 24 hours after MCAO compared to vehicle control mice (p < 0.01). Data are expressed as mean ± SEM (n = 5-6/group). A Two-Way ANOVA with Dunnett’s test was used. All the treatments were randomized for in vivo studies. (D) Representative live/death images of tilorone preconditioning, followed by 3.5 hours of OGD condition, followed by 24 hours reperfusion in mature neurons. 3 µM Ti preconditioning provided neuroprotection against OGD in mature mouse neurons.
MTT assay results showing neuroprotection, as % cell viabilility. 3 µM Ti preconditioning protected against OGD-induced death in mature neurons. As a positive control, MK-801 showed protection in OGD-induced death in mature neurons. A two-tailed t test was used. Data are expressed as mean ± SEM (n = 5). ***p < 0.001, **p < 0.01 compare to OGD control group.
Tilorone reduced HIF-regulated gene expression in the brain, liver, and kidney of mice
Prior studies from our lab identified tilorone as a novel and robust activator of HIF-driven luciferase expression in hippocampal neuroblasts (Ratan et al., 2008; Karuppagounder et al., 2018). To determine whether tilorone-mediated protection in mice is correlated with HIF-dependent gene expression in brain and other tissues, we monitored levels of mRNAs associated with hypoxic adaptation using a commercially developed Mouse Hypoxia Signaling Array (Qiagen). Heat map of genes associated with hypoxic adaptation showed that many established HIF-regulated genes were not induced 12 hours after IP administration of tilorone (Figure 2A). On the contrary, many known HIF targets were downregulated by tilorone (e.g., Vegf, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (Pfkfb4), and phosphoglycerate kinase 1 (Pgk1)). Of known HIF target genes, Vegf, lactate dehydrogenase (Ldh), and erythropoietin (Epo) were selected for qPCR measurements to verify the results obtained with the HIF array. Analysis of mRNA levels at various time points after tilorone treatment (12 to 24 hours) confirmed that in the brain and liver, Vegf, Ldh, and Epo expression were significantly downregulated (Figure 2B). By contrast, in the spleen and kidney, Vegf and Ldh expression were not significantly changed, whereas Epo was downregulated in the kidney (Figure 2C). Consistent with our results in brain, we also found that tilorone downregulated Vegf and Ldh gene expression in HT22 hippocampal neuroblast cells (Supplementary Figure 2A,B). By contrast, the selective HIF stabilizer, adaptaquin, upregulated Vegf and Ldh gene expression at 4-12 hours after treatment (Karuppagounder et al., 2016) (Supplementary Figure 2C, D). These results suggest that tilorone downregulates HIF-related gene expression in an organ specific manner and are consistent with the possibility that suppression of hypoxic adaptation including VEGF may be a critical feature of tilorone-mediated neuroprotection. Indeed, VEGF has been shown to activate cell death signaling (Narasimhan et al., 2009), to increase blood brain barrier permeability and to worsen outcomes after stroke, particularly in the setting of known stroke comorbidities (Geiseler and Morland, 2018).
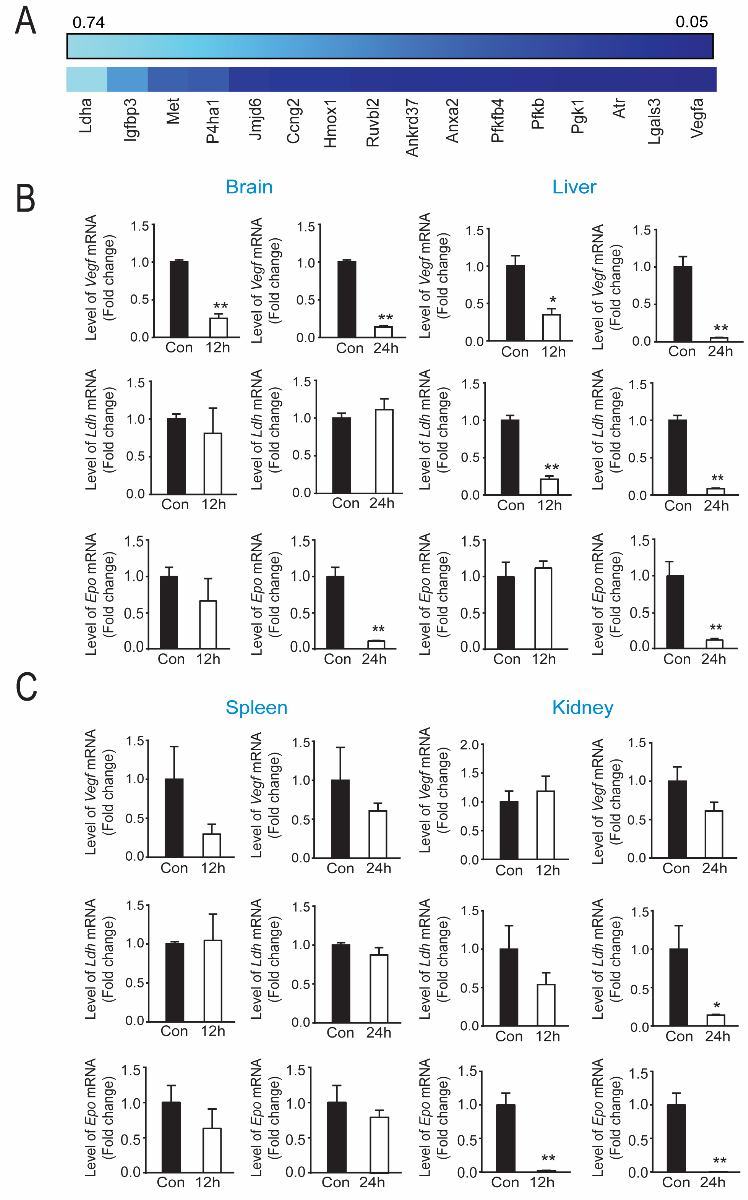
In a new window | Download PPT
Figure 2: Protective levels of tilorone downregulates hypoxia inducible factor (HIF) associated gene expression in vivo. (A) PCR array showed that tilorone treatment (12 h) downregulates HIF target gene expression in mouse brain, compared to the vehicle control. Data are shown as a heat map reflecting triplicate experiments with lighter blue reflecting less and darker blue reflecting more downregulation. (B) Vegf mRNA levels were down-regulated by tilorone treatment in the brain and liver at 12 (p < 0.01) (B) and 24 hours (p < 0.01). Ldh gene expression was unaltered by tilorone treatment in the brain at 12 and 24 hours, but was down-regulated by tilorone treatment in the liver significantly at 12 (p < 0.01) and 24 (p < 0.01) hours. Epo gene expression was unaltered by tilorone treatment in the brain or liver at 12 hours but was reduced significantly (p < 0.01) at 24 hours. (C) Vegf gene expression was not significantly downregulated by tilorone treatment in the spleen and kidney at 12 and 24 hours. We observed no change in Ldh gene expression in the spleen but observed significant reduction in the kidney (p < 0.05) at 24 hours. Epo gene expression was unaltered by tilorone treatment in the spleen but reduced significantly in the kidney at 12 and 24 hours (p < 0.05). qPCR data are expressed as mean ± SEM (n = 3). A two-tailed t test was used. All the treatments were randomized for in vivo studies.
Tilorone induces Type I IFN-associated expression in the brain, liver, spleen, and kidney of mice
Although our original rationale for examining tilorone in stroke models was its ability to robustly drive a hypoxia-driven reporter, tilorone was developed for clinical use as an orally active IFN inducer. Indeed, prior studies have linked type I IFN activation to ischemic-preconditioning (Vartanian et al., 2015; Gesuete et al., 2016). Accordingly, we next examined the effects of tilorone on IFN-associated gene expression. A heat map of IFN-associated genes in brain using an IFN array showed that 12 hours following systemic administration of tilorone, numerous genes in this program are significantly upregulated (Figure 3A). IFN regulatory factor-3 (Irf3), Ifit1, and IFN regulatory factor-5 (Irf5) were selected to validate the array results with qPCR. qPCR analysis verified that Ifit1 mRNA levels were significantly upregulated by tilorone (100 mg/kg; IP) in the brain, liver, spleen, and kidney 12 and 24 hours following treatment (Figure 3B). A 300-fold increase in Ifit1 expression was observed in lysates from brain 12 hours ater treatment, which escalated to a 400-fold increase 24 hours after treatment as compared to the control group. In the liver, Ifit1 was increased by nearly 400-fold 12 hours (Figure 3B) following tilorone treatment. Moreover, Ifit1 gene expression was upregulated about 900-fold in the kidney and 500-fold in the spleen compared to control group after tilorone injection (Figure 3B). mRNA levels for Irf3 and Irf5, transcription factors that can drive IFN synthesis and by extension, other IFN associated genes were also upregulated by tilorone although their pattern of induction was distinct in different organs. Irf3 message was increased significantly in kidney 6 hours (data not shown) and 12 hours after treatment but not in the brain and spleen (Supplementary Figure 3A). By contrast, message for Ir5 was significantly increased by tilorone in the brain and liver 12 hours following tilorone compared to the control group, whereas it did not change significantly in the spleen and kidney (Supplementary Figure 3B). To determine whether changes in message induced by tilorone could be associated with increased protein levels we performed immunoblots using a specific antibody to Ifit1 in lysates from distinct organs at distinct time points after tilorone treatment. We observed a significant increase in Ifit1 protein expression at 6, 12, and 24 hours in the brain, liver, and spleen (Figure 3C), and in the kidney at 24 hours compared to the control group (Figure 3C). Together, our findings suggest that tilorone preconditioning is correlated with significant increases in message and protein levels of genes associated with the antiviral IFN stress response, and unexpected decreases in message levels of hypoxia inducible genes in multiple organs.
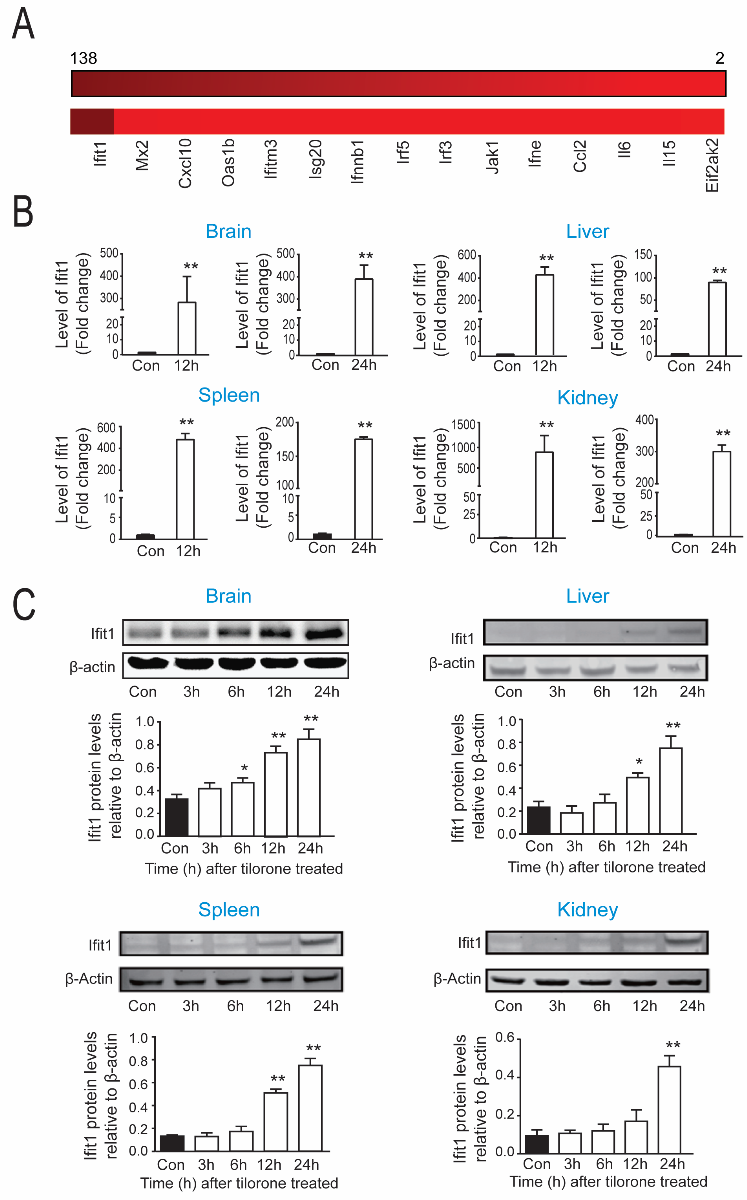
In a new window | Download PPT
Figure 3: Tilorone preconditioning is correlated with increases in antiviral IFN stress response gene expression. (A) IFN associated PCR array showing that tilorone treatment (12 h) upregulated IFN-associated genes in mouse brain compared to the control group. Data were performed as heat map from triplicate experiments. (B) qPCR analysis of Ifit1 gene expression showed upregulation by tilorone in brain at 12 (p < 0.05) and 24) hours (p < 0.01. Additionally, Ifit1 gene expression was significantly upregulated by tilorone treatment in the liver, spleen, and kidney at 12 and 24 (p < 0.01) hours. (C) Ifit1 protein expression in mice. Representative images of western blot showing Ifit1 protein levels from mouse brain, liver, spleen, and kidney at distinct times after IP injection of tilorone . Ifit1 protein induced in brain by systemic tilorone at 6 (p < 0.05), 12 (p < 0.01) and 24 (p < 0.01) hours. Ifit1 protein was also upregulated in the spleen, kidney, and liver. Data are expressed as mean ± SEM (n = 3). A One-Way ANOVA with Dunnett's multiple comparisons test was used.
Tilorone preconditioning requires a non-CNS site of action
Prior studies have suggested that preconditioning is a multi-organ phenomenon (Packard et al., 2012b). To determine whether tilorone preconditioning can occur via direct effects on the CNS, we performed an intracerebroventricular (ICV) injection of tilorone using a range of doses (10 to 200 micrograms) and examined its effect on Ifit1 mRNA, our most upregulated gene in the IFN-gene signature. A range of doses failed to increase Ifit1 mRNA levels (Figure 4A). As expected from these results, injection of tilorone into the ventricles 24 hours before MCAO had no effect on infarct size and behavior (Figure 4B). These studies suggest that a non-CNS site of action is essential to a) precondition the brain against stroke and b) to achieve induction of IFN-associated genes message levels in brain. The correlation of Ifit1 increases in brain only with protective routes of administration suggested that forced expression of Ifit1, our most upregulated gene in the CNS, would protect neurons from ischemic injury. As expected, forced expression of Ifit1 in HT22 hippocampal neuroblasts (Figure 4C) induced resistance to OGD (Figure 4D).

In a new window | Download PPT
Figure 4: Intracerebroventricular (ICV) administration of tilorone does not reduce infarct size. (A) Ifit1 gene expression in brain was unaltered by a range of tilorone doses 12 hours following ICV injections. Tilorone ICV treatment in MCAO mice model schematic protocol. (B) ICV preconditioning of 100 µg Tilorone did not reduce the infarct size in the tilorone treated group compared to vehicle group as shown. Representative images of brain sections after TTC staining 24 hours after 45 min MCAO induced transient ischemia. Aggregate infarct volumes are shown as the cortex and hemisphere. Data are expressed as means ± SEM. (C) Ifit1 overexpression protected against OGD-induced death in hippocampal neuroblast HT22 cells. A representative image of western blot showing IFIT1 protein levels in HT22 cells expressing control or M- Ifit1 vector. Ifit1 protein was upregulated only in M-Ifit1 expressing cells (p < 0.01). (D) Upregulation of Ifit1 induced protection (p < 0.01) against OGD-induced death in HT22 cells, measured by MTT assay. Protein expression data are expressed as mean ± SEM (n = 4). A One-Way ANOVA with Dunnett's multiple comparisons test was used.
Tilorone preconditions transient but not permanent ischemia in mice
The filament occlusion model we utilize involves transient occlusion. Our prior studies in rats suggested that tilorone can protect against permanent ischemic damage in a filament occlusion model (Ratan et al., 2008). To examine whether tilorone could protect against permanent ischemia in a model of photothrombosis, we pretreated mice with tilorone 24 hours prior to inducing stroke using a laser coupled to a fiber-optic cable mounted on the head of a mouse (Figure 5A). Activation of the mounted photodiode laser following systemic delivery of rose bengal allows one to simulate distal MCAO stroke in the awake mouse (Seto et al., 2014). In contrast to our prior results in rats, tilorone failed to reduce infarct size or improve behavior (Figure 5B) in a photothrombotic mouse model.
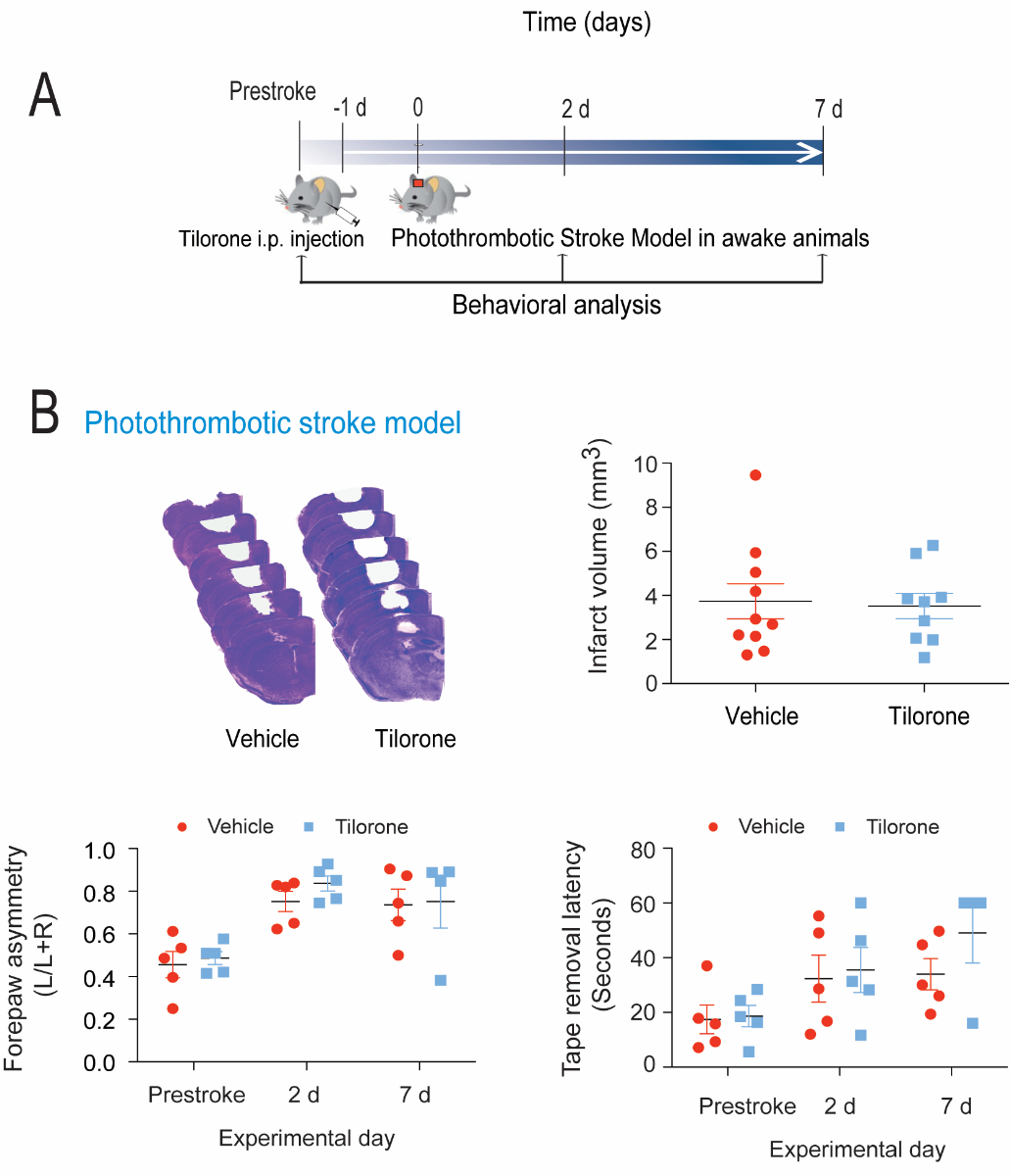
In a new window | Download PPT
Figure 5: Tilorone preconditioning did not reduce infarct volume or behavioral deficits in a photothrombotic stroke model. (A) Tilorone treatment in awake mice stroke model protocol. (B) Preconditioning with 100 mg/kg tilorone did not reduce the infarct volume. Representative images of brain sections (cresyl violet staining) seven days after stroke. Collective infarct volumes are shown. Mice were injected intraperitoneally with 100 mg/kg tilorone 24 hours prior to stroke (n = 9 to 10/group). Data are expressed as means ± SEM. A two-tailed t test was used. Tilorone preconditioning did not improve the corner test score and tape removal task following permanent stroke.
Discussion
Ischemic preconditioning is a substantiated strategy for identifying mechanisms of brain resilience with promise for protecting against surgery-induced damage, transient ischemic attack, subarachnoid-related vasospasm, and cardiac bypass related stroke. Here, we show that tilorone, a drug currently used in humans in Europe, can significantly induce resilience to transient ischemic stroke in mice when delivered prior to stroke. While we do not know whether it is effective post-stroke, its safe use in other countries as an orally effective, IFN inducer suggests that its discontinuation in the United States may have been premature and should be reconsidered. Indeed a recent study showed that a six week course of oral tilorone twice a year in those with heart failure, reduces viral infections and improves cardiac outcomes (Budnevsky et al., 2019).
To induce optimal neuroprotection, tilorone must be delivered systemically but its effects are correlated with induction of antiviral, IFN-associated genes in the brain and the periphery. Indeed, we found that one of the interferon-associated genes, Ifit1, was induced several hundred-fold in the periphery and the brain. Ifit1 is associated with suppression of translation of not only viral RNAs but also of translation in general (Diamond, 2014; Fensterl and Sen, 2015). As translation is metabolically expensive, Ifit1 would be expected to contribute, along with other IFN-associated genes, in diminishing metabolic demand in the setting of ischemia where metabolic supply is reduced. Indeed, we found that Ifit1 overexpression alone was capable of significantly reducing cell death in hippocampal neuroblasts induced by OGD. Although we found protection in neurons, our data do not provide concrete data on when relative to stroke or the precise cell types where IFN-associated genes are induced inside and outside of the nervous system, and whether they are necessary for neuroprotection. The current study sets the table for studies using transgenic mice that will determine the causal role that IFN genes play in tilorone-mediated neuroprotection.
Prior studies from the Stenzel-Poore and Weinstein laboratories have implicated IFN-associated genes in preconditioning-induced protection of brain parenchyma and the blood brain barrier following focal ischemia (Marsh et al., 2009; Stevens et al., 2011; Vartanian et al., 2011; Gesuete et al., 2012; Gesuete et al., 2014). Specifically it was shown that agonists of toll like receptor 4 (TLR4) (LPS), TLR7 (Gardiquimod), and TLR9 (Poly ICLC) reprogram TLR4 and TLR2 prodeath signaling, likely in macrophages, post stroke and reduce infarct size by 35-40% (Stevens et al., 2008; Vartanian et al., 2011; Leung et al., 2012; Packard et al., 2012a; Wang et al., 2015). This reprogramming is associated in each case with the induction of IFN-associated genes, some of which lie upstream of IFNα and IFNβ (e.g. IFN3 and IFN7), and some of which lie downstream of these ligands via activation of cognate IFN receptors (e.g., OAS, IFIT1). Of note, only TLR7-mediated reductions in infarct size with Gardiquimod was shown to depend on the IFN receptor (Leung et al., 2012). We have not measured serum IFN levels in this study, but initial studies nearly 50 years ago showed that tilorone can significantly induce serum IFN levels above those induced by poly dIdC (Stringfellow and Glasgow, 1972). Tilorone has also been shown to affect the immune cells such as NK cells, cytotoxic T cells, and neutrophil cells, which can cross the blood brain barrier (Hermanowicz and Nawarska, 1984; Sharapova et al., 2019). Indeed, although we have not compared tilorone to LPS, gardiquomol, or poly dIdC in our experiments, the magnitude of the reduction in infarct size in response to 45 minutes of transient ischemia by tilorone (70-80%, Figure 1) (Ratan et al., 2008) appears equal or greater than with these established TLR agonists. One of the potential reasons for this difference could be that tilorone not only enhances IFN-associated gene expression, it also, unexpectedly and in contrast to our prior published data, diminishes HIF-regulated gene expression, specifically VEGF. HIF and VEGF have been shown to have deleterious effects acutely via effects on BBB permeability as well as on cell death and infarct size (Narasimhan et al., 2009). These studies would predict, as we found here, that an agent that downregulates HIF function generally and VEGF in particular could actually reduce infarction.
Tilorone’s ability to influence multiple downstream programs begs the question of what is its precise target of action. The ability of tilorone to protect when delivered systemically as opposed to intraventricularly points to a multi-organ mechanism of action. While it is formally possible that the requirement for systemic delivery represents a requirement that tilorone is “activated” via first-pass metabolism in the liver, we believe this is unlikely as we have found that tilorone modulates gene expression (Ratan et al., 2008) and induces neuroprotection (Figure. 1D) when delivered to cells/mature neurons in vitro where first pass metabolism does not occur.
Tilorone’s ability to drive IFN responses appears to depend on its predilection to intercalate AT rich regions of DNA (Nishimura et al., 2007). How this intercalation could lead to a robust antiviral response has not been readily demonstrated, but recent elucidation of a stress response to cytoplasmic DNA accumulation that culminates in IFN induction offers a testable model (Karuppagounder et al., 2018). It is now well established that damage in the nucleus or the mitochondria can lead to the release of double-stranded DNA (dsDNA) in the cytoplasm (Hartlova et al., 2015). In this context, dsDNA represents a damage associated molecular pattern (DAMP) that is capable of triggering innate immunity including antiviral responses. In this scheme, cytosolic DNA binds to an enzyme, cGAS (cAMP-AMP synthase) and leads to increases levels of cGAMP then acts as a ligand for the endoplasmic reticulum -resident protein stimulator of interferon genes (STING). Activated STING recruits the serine/threonine kinase TBK1 and IFN regulatory factor 3 or 7. TBK1 phosphorylates these transcription factors allowing their dimerization and translocation to the nucleus where they can activate IFNα and IFNβ. A model consistent with tilorone’s ability to bind dsDNA is that it stabilizes cytosolic DNA and prevents it from being degraded by the endonuclease, TREX (Karuppagounder et al., 2018). Future studies will clarify the precise site of tilorone’s action and mechanism of action. Whatever the precise mechanism, tilorone’s avid use in other countries as an antiviral drug along with its robust effects in rats and mice in preventing infarction or reducing edema suggests that the pursuit of analogs that are safe and well tolerated could offer near term opportunities for creating resistance to CNS ischemia in circumstances where there is time to prevent incipient damage.
Sources of Funding
This work was funded with support from the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the Burke Foundation, the Sperling Center for Hemorrhagic Stroke Recovery at the Burke Neurological Institute, and the NIH (grant P01 NIA AG014930, project 1, to R.R.R.). We also thank the funding support of the Natural Science Foundation of China (grant number 81622050 & 81873209) and the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (grant number 2017BT01Y036).
Disclosures
None.
References
Yujia Zhai1,2*
1Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou 510632, China. 2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Yingxin Chen2*
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Saravanan S. Karuppagounder2*
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Amit Kumar2
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Nandini Kundu2
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Ishraq Alim2
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
Stephanie Taylor3
3Division of Medical Sciences, University of Victoria, Victoria, BC, Canada.
Craig E. Brown3
3Division of Medical Sciences, University of Victoria, Victoria, BC, Canada.
Xiang Luo1
1Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou 510632, China.
Yi-Fang Li1
1Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou 510632, China.
Dawei Lian4
4School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou 51006, China.
Yang Chen4
4School of Chinese Materia Medica, Guangzhou University of Chinese Medicine, Guangzhou 51006, China.
Hilda Ahnstedt5
5Department of Neurology, University of Texas Medical School at Houston, USA.
Louise D. McCullough5
5Department of Neurology, University of Texas Medical School at Houston, USA.
Rong-Rong He1
1Guangdong Engineering Research Center of Chinese Medicine & Disease Susceptibility, College of Pharmacy, Jinan University, Guangzhou 510632, China.
Rajiv R. Ratan2
2Burke Neurological Institute and Brain and Mind Research Institute, Weill Cornell Medicine.
*These authors contributed equally to this article.
Corresponding author:
Rajiv R. Ratan
Email: rrr2001@med.cornell.edu
.png)
In a new window | Download PPT
Figure 1: Pre-treatment with tilorone can precondition against stroke-induced damage and behavioral dysfunction in mice. (A) The chemical structure of tilorone dihydrochloride. Tilorone (Ti) treatment and the MCAO model protocol. (B) Representative images of brain sections after TTC staining 24 hours after transient MCAO. Preconditioning of 100 mg/kg Ti (n = 10 mice/group) significantly reduced infarct size 24 hours after MCAO. Infarct volumes shown are the cortex (p < 0.05 for Ti compared to control) and hemisphere infarct volumes. Data are expressed as mean ± SEM. Students t test was used to calculate the significance. All the treatments were randomized for in vivo and treatment group assignments. (C) Tilorone improved function as measured by the corner task assessed 24 hours after MCAO in both the 50 mg/kg (Ti L) and 100 mg/kg (Ti H) dose groups compared to vehicle group (p < 0.01 for both doses of Ti compared to control). Tilorone also improved the tape removal task, measured 24 hours after MCAO in both the 50 mg/kg (Ti L) and 100 mg/kg (Ti H) dose groups compared to vehicle control (p < 0.05 for Ti H vs. control, p < 0.01 for Ti L vs. control). Tilorone (100 mg/kg) preconditioning improved the total reach time in the pole test measured 24 hours after MCAO compared to vehicle control mice (p < 0.01). Data are expressed as mean ± SEM (n = 5-6/group). A Two-Way ANOVA with Dunnett’s test was used. All the treatments were randomized for in vivo studies. (D) Representative live/death images of tilorone preconditioning, followed by 3.5 hours of OGD condition, followed by 24 hours reperfusion in mature neurons. 3 µM Ti preconditioning provided neuroprotection against OGD in mature mouse neurons.
MTT assay results showing neuroprotection, as % cell viabilility. 3 µM Ti preconditioning protected against OGD-induced death in mature neurons. As a positive control, MK-801 showed protection in OGD-induced death in mature neurons. A two-tailed t test was used. Data are expressed as mean ± SEM (n = 5). ***p < 0.001, **p < 0.01 compare to OGD control group.

In a new window | Download PPT
Figure 2: Protective levels of tilorone downregulates hypoxia inducible factor (HIF) associated gene expression in vivo. (A) PCR array showed that tilorone treatment (12 h) downregulates HIF target gene expression in mouse brain, compared to the vehicle control. Data are shown as a heat map reflecting triplicate experiments with lighter blue reflecting less and darker blue reflecting more downregulation. (B) Vegf mRNA levels were down-regulated by tilorone treatment in the brain and liver at 12 (p < 0.01) (B) and 24 hours (p < 0.01). Ldh gene expression was unaltered by tilorone treatment in the brain at 12 and 24 hours, but was down-regulated by tilorone treatment in the liver significantly at 12 (p < 0.01) and 24 (p < 0.01) hours. Epo gene expression was unaltered by tilorone treatment in the brain or liver at 12 hours but was reduced significantly (p < 0.01) at 24 hours. (C) Vegf gene expression was not significantly downregulated by tilorone treatment in the spleen and kidney at 12 and 24 hours. We observed no change in Ldh gene expression in the spleen but observed significant reduction in the kidney (p < 0.05) at 24 hours. Epo gene expression was unaltered by tilorone treatment in the spleen but reduced significantly in the kidney at 12 and 24 hours (p < 0.05). qPCR data are expressed as mean ± SEM (n = 3). A two-tailed t test was used. All the treatments were randomized for in vivo studies.

In a new window | Download PPT
Figure 3: Tilorone preconditioning is correlated with increases in antiviral IFN stress response gene expression. (A) IFN associated PCR array showing that tilorone treatment (12 h) upregulated IFN-associated genes in mouse brain compared to the control group. Data were performed as heat map from triplicate experiments. (B) qPCR analysis of Ifit1 gene expression showed upregulation by tilorone in brain at 12 (p < 0.05) and 24) hours (p < 0.01. Additionally, Ifit1 gene expression was significantly upregulated by tilorone treatment in the liver, spleen, and kidney at 12 and 24 (p < 0.01) hours. (C) Ifit1 protein expression in mice. Representative images of western blot showing Ifit1 protein levels from mouse brain, liver, spleen, and kidney at distinct times after IP injection of tilorone . Ifit1 protein induced in brain by systemic tilorone at 6 (p < 0.05), 12 (p < 0.01) and 24 (p < 0.01) hours. Ifit1 protein was also upregulated in the spleen, kidney, and liver. Data are expressed as mean ± SEM (n = 3). A One-Way ANOVA with Dunnett's multiple comparisons test was used.

In a new window | Download PPT
Figure 4: Intracerebroventricular (ICV) administration of tilorone does not reduce infarct size. (A) Ifit1 gene expression in brain was unaltered by a range of tilorone doses 12 hours following ICV injections. Tilorone ICV treatment in MCAO mice model schematic protocol. (B) ICV preconditioning of 100 µg Tilorone did not reduce the infarct size in the tilorone treated group compared to vehicle group as shown. Representative images of brain sections after TTC staining 24 hours after 45 min MCAO induced transient ischemia. Aggregate infarct volumes are shown as the cortex and hemisphere. Data are expressed as means ± SEM. (C) Ifit1 overexpression protected against OGD-induced death in hippocampal neuroblast HT22 cells. A representative image of western blot showing IFIT1 protein levels in HT22 cells expressing control or M- Ifit1 vector. Ifit1 protein was upregulated only in M-Ifit1 expressing cells (p < 0.01). (D) Upregulation of Ifit1 induced protection (p < 0.01) against OGD-induced death in HT22 cells, measured by MTT assay. Protein expression data are expressed as mean ± SEM (n = 4). A One-Way ANOVA with Dunnett's multiple comparisons test was used.

In a new window | Download PPT
Figure 5: Tilorone preconditioning did not reduce infarct volume or behavioral deficits in a photothrombotic stroke model. (A) Tilorone treatment in awake mice stroke model protocol. (B) Preconditioning with 100 mg/kg tilorone did not reduce the infarct volume. Representative images of brain sections (cresyl violet staining) seven days after stroke. Collective infarct volumes are shown. Mice were injected intraperitoneally with 100 mg/kg tilorone 24 hours prior to stroke (n = 9 to 10/group). Data are expressed as means ± SEM. A two-tailed t test was used. Tilorone preconditioning did not improve the corner test score and tape removal task following permanent stroke.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 11338 | 66 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA