International bi-monthly journal of cell signaling, tissue protection, and translational research.
Extracellular vesicles - mediating and delivering cardioprotection in acute myocardial infarction and heart failure
Aishwarya Prakash1, Gustavo E. Crespo-Avilan1,2,3, Sauri Hernandez-Resendiz1,2,4, Sang-Ging Ong5,6, Derek J. Hausenloy1,2,7,8,9
Author Affiliations


- 1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
- 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
- 3Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
- 4Tecnologico de Monterrey, Centro de Biotecnologia-FEMSA, Nuevo Leon, Mexico.
- 5Department of Pharmacology, University of Illinois College of Medicine, Chicago, Illinois, United States of America.
- 6Division of Cardiology, Department of Medicine, University of Illinois College of Medicine, Chicago, Illinois, United States of America.
- 7Yong Loo Lin School of Medicine, National University Singapore, Singapore.
- 8The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, UK.
- 9Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Abstract
New treatments are urgently needed to reduce myocardial infarct size and prevent adverse post-infarct left ventricular remodeling, in order to preserve cardiac function, and prevent the onset of heart failure in patients presenting with acute myocardial infarction (AMI). In this regard, extracellular vesicles (EVs) have emerged as key mediators of cardioprotection. Endogenously produced EVs are known to play crucial roles in maintaining normal cardiac homeostasis and function, by acting as mediators of intercellular communication between different types of cardiac cells. Endogenous EVs have also been shown to contribute to innate cardioprotective strategies such as remote ischemic conditioning. In terms of EV-based therapeutics, stem cell-derived EVs have been shown to confer cardioprotection in a large number of small and large animal AMI models, and have the therapeutic potential to be applied in the clinical setting for the benefit of AMI patients, although several challenges need to be overcome. Finally, EVs may be used as vehicles to deliver therapeutics to the infarcted heart, providing a potential synergist approach to cardioprotection. In this review article, we highlight the various roles that EVs play as mediators and deliverers of cardioprotection, and discuss their therapeutic potential for improving clinical outcomes following AMI.
Keywords: Cardioprotection, extracellular vesicles, heart failure, drug delivery, acute myocardial infarction, exosomes
Abstract
New treatments are urgently needed to reduce myocardial infarct size and prevent adverse post-infarct left ventricular remodeling, in order to preserve cardiac function, and prevent the onset of heart failure in patients presenting with acute myocardial infarction (AMI). In this regard, extracellular vesicles (EVs) have emerged as key mediators of cardioprotection. Endogenously produced EVs are known to play crucial roles in maintaining normal cardiac homeostasis and function, by acting as mediators of intercellular communication between different types of cardiac cells. Endogenous EVs have also been shown to contribute to innate cardioprotective strategies such as remote ischemic conditioning. In terms of EV-based therapeutics, stem cell-derived EVs have been shown to confer cardioprotection in a large number of small and large animal AMI models, and have the therapeutic potential to be applied in the clinical setting for the benefit of AMI patients, although several challenges need to be overcome. Finally, EVs may be used as vehicles to deliver therapeutics to the infarcted heart, providing a potential synergist approach to cardioprotection. In this review article, we highlight the various roles that EVs play as mediators and deliverers of cardioprotection, and discuss their therapeutic potential for improving clinical outcomes following AMI.
Keywords: Cardioprotection, extracellular vesicles, heart failure, drug delivery, acute myocardial infarction, exosomes
Introduction
Despite current best therapy, acute myocardial infarction (AMI) and the heart failure (HF) that often follows are among the leading causes of death and disability worldwide. The detrimental effects of AMI arise from the effects of acute ischemia/reperfusion injury (IRI) on the myocardium, the results of which include cardiomyocyte death, myocardial inflammation and fibrosis, leading to adverse post-infarct left ventricular (LV) remodeling (dilatation and thinning of the LV and impaired contractile function), which predisposes the patient to develop HF (Turer and Hill, 2010; Minicucci et al., 2011; Yang, 2018). As such, novel cardioprotective therapies are urgently needed to reduce myocardial infarct (MI) size, and prevent adverse post-infarct LV remodeling, in order to preserve cardiac function, and avert the onset of HF. In this regard, extracellular vesicles (EVs) have been intensively investigated for their roles as mediators of intercellular communication within the heart, effectors of cardioprotection, and potential delivery vehicles for cardioprotective therapeutics (Barile et al., 2017; Li et al., 2019; Rezaie et al., 2019).
Three main types of EVs have been described: Exosomes (30-200 nm in diameter) which are EVs of endosomal origin; microvesicles (50-1000 nm in diameter), which originate from direct budding of plasma membranes from injured or transformed cells; and apoptotic bodies (50–5000 nm in diameter), which arise as fragments of cells undergoing programmed death (EL Andaloussi et al., 2013; Atkin-Smith et al., 2015; Doyle and Wang, 2019). Exosomes are bi-lipid membrane vesicles that were first described as providing a garbage function for disposal of unwanted transferrin receptors in maturing reticulocytes (Pan and Johnstone, 1983). However, exosomes are now recognized to mediate intercellular communication through the transfer of proteins, lipids, and RNA, with the content varying according to cell type and prevailing conditions. They are formed in the multi-vesicular body (MVB) by the invagination of the endosomal membrane, and are released into the extracellular space by fusion of the MVB with the plasma membrane (Fevrier and Raposo, 2004). Uptake of EVs by the recipient cell can take place through a variety of mechanisms such as endocytosis, direct plasma membrane fusion, phagocytosis, and micropinocytosis (Mulcahy et al., 2014; French et al., 2017). Of the 3 types of EVs, exosomes have been investigated as mediators of cardioprotection against AMI and adverse post-infarct LV remodeling, and their biological properties (non-immunogenic, small, easily taken up by target cells), make them ideal vehicles for delivery of therapeutics and genes to the heart. In this article, we highlight the role of EVs, especially exosomes, as key mediators of intercellular communication within the heart, effectors of cardioprotection, and as cardiac-homing/targeting delivery vehicles of therapeutics to the infarcted heart (Figure 1). We also discuss the challenges and obstacles facing the translation of EV-based therapy into the clinical setting for the benefit of AMI patients to prevent HF and improve clinical outcomes.

In a new window | Download PPT
Figure 1: Extracellular vesicles and cardioprotection. Extracellular vesicles (EVs) are known to act a mediators of endogenous cardioprotection by mediating intercellular communication between cardiomyocytes and endothelial cells (ECs), fibroblasts, pericytes, and immune cells. In addition, plasma EVs have been shown to mediate the cardioprotection elicited by endogenous strategies such as ischemic preconditioning (IPC) or remote ischemic conditioning (RIC). Stem cell-derived EVs from mesenchymal stromal cells (MSC), cardiac progenitor cells (CPC), and cardiosphere derived cells (CDC) have been shown to reduce myocardial infarct size and to prevent adverse left ventricular remodeling in small and large animal infarct models through a variety of mechanisms including cytoprotection, anti-apoptotic effects, metabolic effects, immunomodulation, increasing autophagy, and enhancing angiogenesis. Stem cell-derived EVs can be used as drug delivery systems to deliver cardioprotective drugs to the ischemic heart following acute myocardial infarction. Finally, addition of cardiac homing/targeting peptides to stem cell-derived EVs can target them to the ischemic heart, thereby increasing bioavailability and cardioprotective efficacy.
Endogenous EVs as mediators of intercellular communication within the heart
The adult mammalian heart is composed of a variety of cells including cardiomyocytes, fibroblasts, endothelial cells (EC), perivascular cells, neuronal cells, immune cells, and cardiac progenitor stem cells (Zhou and Pu, 2016; Amini et al., 2017). To ensure the proper working of the heart, these different types of cells have to function with precise coordination and regulation. Intercellular communication is essential to facilitate well-balanced functioning of the heart. One of the main methods to ensure intercellular communication is the secretion of EVs (Sluijter et al., 2014). EVs act as a means of crosstalk between the cells during normal physiological and in pathological conditions (Figure 1). However, there are only limited studies exploring the intercellular communication via EVs in the context of cardioprotection (Giricz et al., 2014; Garcia et al., 2015).
EVs as intercellular mediators of cardioprotective effects
Garcia et al. (2015) found that glucose restriction in H9C2 rat cardiomyoblasts caused an increase in the release of exosomes and modifications in their cargo, which promoted angiogenesis in ECs. Exosomes released from glucose starved cardiomyocytes were found to overexpress miRNA-17, 19a, 19b, 20a, 30c, and 126, which were capable of inducing angiogenesis when internalized by the ECs. Also, EVs secreted by cardiomyocytes under glucose-starvation conditions showed an increase in protein content associated with metabolic signaling pathways for energy acquisition, while EVs from physiological cardiomyocytes had higher protein content associated with vesicle trafficking. Rodrigues et al. (2017) examined exosomes released by primary cultures of cardiomyocytes and H9c2 myoblasts exposed to simulated ischemia and found upregulation of miR-222 and miR-143 in both cases, which are known to induce cell migration and tube formation in ECs. Van Balkom et al. (2013) studied endothelial cell crosstalk, and found that ECs released exosomes rich in miR-214, which played a role in promoting angiogenesis, and preventing cell cycle arrest in recipient ECs. It was also found that the co-culture of ECs with activated pericytes promoted their angiogenic properties (Mayo and Bearden, 2015), providing evidence that exosomes are a necessary signaling mechanism for promoting angiogenesis in pericytes by chemical inhibition of ceramide-dependent exosome secretion.
Wang et al. (2015) studied the EVs released from murine cardiac fibroblasts and cardiac fibroblasts-derived induced pluripotent stem cells (iPS). They showed that iPS exosomes contained high levels of miR-21 and miR-210 in comparison to cardiac fibroblast exosomes. MiR-21 has been recently shown to be an anti-apoptotic and pro-survival factor while miR-210 has been shown to reduce reactive oxygen species (ROS) levels, and cell death on exposure to oxidative stress (Mutharasan et al., 2011; Kura et al., 2020). An interesting study by Borosch et al. (2017) showed that cardiac fibroblasts that have undergone in vitro preconditioning (hypoxia or isoflurane) release cardioprotective EVs that induce cell migration and fibroblast proliferation. This led to the conclusion that the same cardiac fibroblasts when exposed to different stimuli can release EVs with varying compositions and functionality (Borosch et al., 2017).
Zhang et al. (2012) found that cardiomyocytes secreted EVs rich in heat shock protein 20 (Hsp20), which functioned as a mediator for angiogenesis through direct interaction with the vascular endothelial growth factor (VEGF) receptor (VEGFR2) on the endothelial cells. They subjected 12-week old mice to acute myocardial IRI and found a 3.4 fold increase in Hsp20 serum levels in mice, which had undergone myocardial IRI compared to sham controls. Along with mediating angiogenesis, Hsp20 also confers cardioprotection by inhibiting cell death through multiple interactions with apoptosis signal-regulating kinase 1 (ASK1), Akt, α - actin, α - actinin, Bax and nuclear factor kappa B (NF-κB) (Fan et al., 2005; Wang et al., 2009). However, in this study not all the observed effects can be confirmed to be due to Hsp20 in the EVs, as this study did not test for EV markers.
Yang et al. (2016) observed exosomes highly enriched in miRNA-30a in the serum of AMI patients in vivo as well as in vitro culture of cardiomyocytes after exposure to hypoxia. From their study, it could be concluded that hypoxia stimulates the release of miRNA-30a rich exosomes, which acts as a means of communication between cardiomyocytes to regulate autophagy.
EVs as intercellular mediators of pathological effects
Apart from influencing cardioprotective effects, EVs have also been shown to induce certain pathological effects such as cardiac hypertrophy and arrhythmias (2019). Bang et al. (2014) studied EVs released by neonatal rat cardiac fibroblasts and found them to be enriched with miR-21-3p. This miRNA was found to mediate hypertrophy in neonatal rat cardiomyocytes. studied EV crosstalk between cardiomyocytes and macrophages and observed that under physiological conditions, EVs secreted by cardiomyocytes maintain a specialized profile of cardiac macrophages including a basal interleukin (IL)-1β and IL-6 expression as well as p38 MAPK and NF-κB activation. But in an ischemic environment, the EVs released by the cardiomyocytes were found to increase VEGF levels in macrophages, which allowed these immune cells to play a proangiogenic role. In addition, compared to EVs secreted by cardiomyocytes under physiological conditions, EVs secreted by cardiomyocytes during ischemia failed to maintain Connexin 43 (Cx43)-mediated macrophage adhesion to cardiomyocytes. The deletion of Cx43 macrophages has been found to cause a delay in atrioventricular conduction (Hulsmans et al., 2017). Thus, the EVs released by cardiomyocytes under ischemic conditions can indirectly contribute to arrhythmias as well as reduce the ability of the macrophages to stimulate phagocytosis, thereby impairing the transition from the inflammatory to reparatory phase after ischemia. The impact of IRI on EV-mediated intercellular crosstalk can be elucidated in this study, where ischemic exposure alters the EVs released by the cardiomyocytes leading to direct implications in macrophage protective properties causing adverse cardiac remodeling.
Endogenous EVs as mediators of acute cardioprotection following AMI
Studies have shown that endogenous plasma EVs can protect the heart against acute myocardial IRI (Vicencio et al., 2015) and may mediate the cardioprotective effects of endogenous strategies such as remote ischemic conditioning (RIC) (Figure 1) (Giricz et al., 2014; Minghua et al., 2018). In RIC, cycles of brief non-lethal ischemia and reperfusion applied to the limb have been shown to reduce MI size following acute myocardial IRI (Chong et al., 2018; Chong et al., 2019). It has been postulated that EVs generated in the limb following the RIC stimulus enter the circulation and convey the cardioprotective signal from the limb to cardiomyocytes in the heart. The first experimental study to report cardioprotection with endogenously produced EVs was by Giricz et al (2014), who showed that direct ischemic preconditioning (IPC, comprising 3x5-min alternating episodes of global ischemia and reperfusion) of isolated perfused ex vivo rat hearts increased the number of EVs in the coronary effluent. Perfusion of naïve isolated perfused rat hearts with the IPC effluent limited MI size, but failed to do so if the EVs were depleted from the IPC effluent (Giricz et al., 2014). Vicencio et al (2015) showed that plasma EVs from rats and healthy volunteers were able to reduce cardiomyocyte death and limit MI size in isolated cardiomyocytes subjected to simulated IRI, and in ex vivo perfused and in vivo rodent hearts subjected to IRI. The endogenous EVs were shown to mediate cardioprotection through a toll-like receptor 4/extracellular signal-regulated kinase 1/2 (Erk1/2)/p38 MAPK/HSP27 signaling pathway in cardiomyocytes (Vicencio et al., 2015).
Although hind-limb RIC in rats was able to increase the number of plasma EVs,, they did not confer greater cardioprotection in EVs isolated from control rats (Vicencio et al., 2015). In contrast, other studies have shown that limb RIC does produce plasma EVs that are able to confer cardioprotection. Minghua et al (2018) showed in a rat model that limb RIC (4x5 min episodes of ischemia and reperfusion) increased circulating levels of plasma EVs compared to sham control, and levels miRNA-24 in EVs were higher with RIC. These EVs were shown to protect H9c2 cells against hydrogen peroxide-induced apoptotic cell death through downregulation of the Bcl-2-like protein 11 (BIM) expression (Minghua et al., 2018). Furthermore, intramyocardial injection of RIC EVs reduced MI size and preserved cardiac function, and this cardioprotective effect was abrogated with antagomirs to miRNA-24 (Minghua et al., 2018). However, no evidence was provided to show that plasma EVs containing miRNA-24 produced by limb RIC were actually taken up by the ischemic heart, and that miRNA-24 in EVs actually contributed to the infarct-limiting effects of limb RIC (Minghua et al., 2018). Repeated episodes of limb RIC applied daily for 28 days, and initiated 4 weeks post-infarction, have been shown to prevent adverse cardiac remodeling, and this chronic limb RIC protocol was able to increase levels of miRNA-29a in plasma EVs, and enhance miRNA-29a expression in the infarcted heart, although the role of EVs as mediators of cardioprotection was not investigated in this study (Yamaguchi et al., 2015). Finally, in a recent study, limb RIC has been reported to generate plasma EVs containing miRNA-21, and these were shown to protect kidneys against sepsis-mediated acute injury by suppressing NF-kB activation and the activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway (Chen et al., 2020). Further studies are needed to identify the cells that release the cardioprotective EVs in the setting of limb RIC - one potential candidate could be endothelial cells of the limb vasculature.
In contrast to the experimental studies implicating a potential role for EVs as intercellular mediators of limb RIC cardioprotection, a recent clinical study in acute ST-segment elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PPCI), failed to demonstrate an increase in the overall numbers of plasma EVs with limb RIC versus control, although they did observe an increase in numbers of granulocyte-derived EVs, the significance of which is not clear (Haller et al., 2020). Similarly, in healthy volunteers, limb RIC did not increase overall numbers of plasma ECVs. These findings are consistent with the CONDI2/ERIC-PPCI trial, which failed to demonstrate any improvement in clinical outcomes (heart failure hospitalization and cardiac death) after one year with limb RIC in STEMI patients treated by PPCI (Hausenloy et al., 2019).
Stem-cell derived EVs as mediators of acute cardioprotection
Stem cell therapy for cardiac repair post-AMI has moved away from the concept of regeneration of new cardiomyocytes to replace dead cells within the infarct zone, and has focused instead on the paracrine cytoprotective effects of stem cell secretome (Gnecchi et al., 2005), in particular its EV component. A number of experimental studies have demonstrated cardioprotection with exosomes derived from a wide variety of stem cells, the most common of which has been mesenchymal stromal cells (Figure 1, Table 1).
Table 1. Major animal studies demonstrating EV cardioprotection and the potential underlying mechanisms of action.
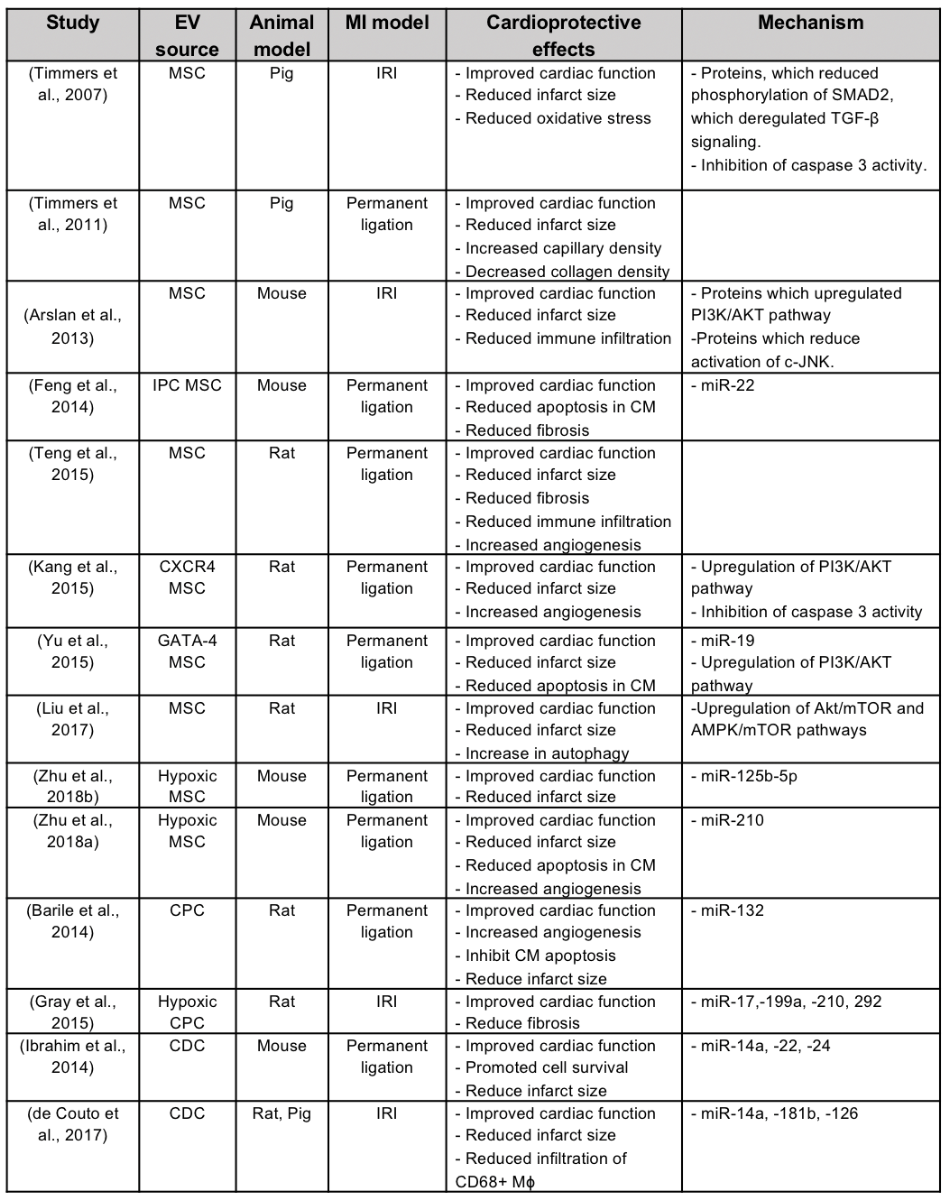
Mesenchymal stromal cell, MSC; cardiac progenitor cell, CPC; cardiosphere derived cells, CDC; ischemic preconditioning, IPC; ischemia/reperfusion injury, IRI; Cardiomyocyte, CM; Chemokine receptor 4, CXCR4; Macrophage, Mφ; Micro RNA, miR.
Mesenchymal stromal cells
Mesenchymal stromal cells (MSCs) are multipotent adult stem cells that reside within the bone marrow microenvironment and can be generated from a variety of cell sources. A number of experimental studies have reported cardioprotective effects of MSC secretome (Gnecchi et al., 2005), and proteomic analyses of the secretome have identified exosomal proteins to be the likely component responsible for conferring cardioprotection (Sze et al., 2007; Timmers et al., 2007). The first study to demonstrate the cardioprotective effects of MSC-derived exosomes was by Lai et al (2010) who showed that exosomes (produced from MSCs derived from human embryonic stem cells [hESC-MSCs]), administered 5-min before reperfusion via tail vein injection in a mouse model of acute myocardial IRI, reduced MI size 24 hrs later. Crucially, cardioprotection was recapitulated in an isolated perfused mouse heart subjected to acute IRI confirming a direct effect of the exosomes on heart tissue (Lai et al., 2010).
A large number of studies have gone on to confirm the acute cardioprotective effects of exogenously applied MSC EVs derived from a variety of sources, and have begun to unravel potential mechanisms underlying their beneficial effects. MSC-derived exosomes are known to contain both proteins, mRNAs and miRNAs, which may, in part, contribute to the cardioprotective effects of exosomes in the setting of AMI. For acute infarct size limitation elicited by EVs administered at the onset of reperfusion, the acute cardioprotective effects of EVs should manifest in the first few minutes of reperfusion, and are therefore most likely to be due to the transfer of cytoprotective proteins to the heart rather than the mRNAs and miRNAs that need time to modify expression of cytoprotective proteins.
Studies have demonstrated a variety of cardioprotective effects with the acute administration of exogenous MSC-derived exosomes including metabolic, anti-apoptotic, and immunomodulatory effects. Proteomic profiling of exosomes from h-ESC-MSCs has identified several proteins, which may mediate acute cardioprotection including: functional 20S proteasomes (the presence of which was associated with a reduction in oxidative-damaged misfolded proteins in a mouse model of AMI) (Lai et al., 2012); enzymes involved with glycolytic ATP production (that can help sustain energy production whilst oxidative phosphorylation is being restore in the reperfused heart) (Li et al., 2012); proteins (such as CD76), which increase extracellular adenosine levels and phosphorylate the cytoprotective PI3K/Akt component of the cytoprotective Reperfusion Injury Salvage Kinase (RISK) pathway, and to inhibit the phosphorylation of the pro-apoptotic protein c-Jun N-terminal kinase (c-Jnk) (Arslan et al., 2013). Liu et al. (2017) found that intramyocardial injection of exosomes produced from bone-marrow-derived MSCs into the infarcted region 5 minutes prior to reperfusion in a rat AMI model, attenuated apoptosis and promoted autophagy via the AMP-activated protein kinase (AMPK)/ mammalian target of rapamycin (mTOR) and Akt-mTOR pathways. In addition, given the immunomodulatory effects of EV, it has been shown that exogenous administration of EVs at the time of reperfusion can prevent the acute inflammatory response of AMI as evidenced by reduced myocardial inflammation (with reduced neutrophil and macrophage infiltration in hearts (Arslan et al., 2013; Zhang et al., 2014, and decreased levels of circulating white blood cells) (Arslan et al., 2013).
Over-expression of particular genes in MSCs have been shown to augment the cardioprotective effect of EVs. Yu et al (2015) over-expressed GATA4 in MSCs to enhance the cardioprotective effects of their EVs and demonstrated that intramyocardial injection of the EVs delivered a variety of anti-apoptotic miRNAs such as miR-19a, which enhanced expression of anti-apoptotic Akt and Erk1/2 proteins, inhibited apoptotic cell death, reduced MI size, and preserved cardiac function in a mouse permanent left arterial descending (LAD) artery ligation model. Overexpression of macrophage inhibitor factor (MIF, a pro-inflammatory cytokine) in bone-marrow derived MSCs has been shown to produce EVs that enhanced cardioprotective efficacy compared to control EVs as evidenced by greater protection of neonatal rat cardiomyocytes from IRI-induced mitochondrial fission and cell death. Intramyocardial injection of the engineered EVs conferred augmented in vivo cardioprotection compared to control EVs as evidenced by inhibition of mitochondrial fragmentation, attenuation of oxidative stress, inhibition of apoptotic cell death, reduced MI size, and preserved cardiac function in a rat permanent ligation MI model (Liu et al., 2020).
Similarly, treatment of MSCs using stimuli such as hypoxia has been reported to enrich EVs in miR-22 (Feng et al., 2014), miR-125b-5p (Zhu et al., 2018b), or miR-210 (Zhu et al., 2018a; Cheng et al., 2020) and augment their cardioprotective effects (reduced MI size, less apoptotic cell death, and preserved cardiac function) following AMI when injected into the infarct border zone in mouse models of permanent LAD ligation. MiR-22 was found to target methyl CpG binding protein (Mecp2) which is known to be upregulated in ischemic hearts (Feng et al., 2014), and miR125b-5p was reported to attenuate the expression of pro-apoptotic genes p53 and BAK1 in cardiomyocytes. Zhu et al. (2018a) investigated the mechanism through which hypoxia increased the expression of miR-210 in MSCs, and implicated hypoxia-inducible factor-1α mediated expression of neutral sphingomyelinase 2, a protein regulator of exosome biogenesis (Trajkovic et al., 2008). In their study, they speculated that miR-210 mediated its cardioreparative effects by inhibiting apoptosis via downregulation of caspase-8 associated protein 2 (Kim et al., 2009) and ephrin A3 (Fasanaro et al., 2008), and promoting angiogenesis by upregulating VEGF (Zeng et al., 2014). Li et al (2020) have shown that intramyocardial injection of EVs produced from bone-marrow-derived stem cells into the peri-infarct zone in a mouse permanent LAD ligation model reduced apoptosis, attenuated inflammation, reduced MI size, and preserved cardiac function by targeting suppressor of cytokine signaling 2 (SOCS2), a known mediator of cardiac injury.
Takov et al (2020) have demonstrated the cardioprotective effects of EVs produced from human fetal amniotic fluid stem cells when intravenously injected 5 min prior to reperfusion in a rat AMI model. Interestingly, cardioprotection was not recapitulated in isolated rat ventricular cardiomyocytes subjected to simulated IRI suggesting an indirect cardioprotective effect of the EVs. Finally the cardioprotective efficacy of EVs derived from different MSC sources were compared by Xu et al (2020) who found that EVs from adipose tissue-derived MSCs had greater cardioprotective efficacy when compared to EVs produced from either umbilical cord or bone marrow-derived MSCs, although the mechanisms underlying this difference was not explored in this study.
Cardiac Progenitor Cells
Cardiac progenitor cells (CPC) are resident cardiac stem cells that contribute to cardiac tissue regeneration after injury. Chen et al (2013) demonstrated that EVs produced by CPCs isolated from mouse heart protected H9c2 myoblasts from oxidative stress by inhibiting caspase 3/7 activation in vitro, and reduced cardiomyocyte apoptosis when administered via intramyocardial injection just after LAD ligation in a mouse AMI model, demonstrating for the first time the cardioprotective effects of CPC-derived EVs. In a subsequent study (Barile et al., 2014), it was shown that EVs produced from CPCs (isolated from human atrial appendage samples), inhibited apoptosis in mouse HL-1 cardiomyocytes and increased tube formation in human umbilical vein endothelial cells. EVs secreted by the CPCs were enriched in miR-210, which inhibited apoptosis by targeting ephrin A3 and PTP1b, and miR-132, which enhanced tube formation in endothelial cells by targeting RasGAP-p120 (Barile et al., 2014). Intramyocardial injection of CPC EVs into the peri-infarct zone 60 min after permanent LAD ligation in a rat AMI model was shown to reduce myocardial apoptosis, enhance angiogenesis and preserve cardiac function (Barile et al., 2014). Gray et al. (2015) also conducted in vivo experiments in a rat model of IRI, and the exosomes secreted by hypoxia-treated CPC (isolated from neonatal rats) were injected into the peri-infarct zone, and were shown to improve cardiac function following AMI, and this beneficial effect was associated with increased levels of miR-17, -199a, -210, and -292, which are known to target genes in the fibrosis pathway.
Cardiosphere Derived Cells
Cardiosphere derived cells (CDCs) are considered to be a type of cardiac progenitor cells that have been obtained from ex vivo heart biopsy specimens. Ibrahim at al. (2014) found that CDC exosomes promoted tube formation in human umbilical vein endothelial cells (HUVEC), stimulated proliferation, and inhibited apoptosis of neonatal cardiomyocytes. Intramyocardial injection of CDC exosomes into the peri-infarct region in a mouse model of permanent ligation reduced MI size and preserved cardiac function confirming the cardioreparative effects of CDC-derived exosomes in vivo (Ibrahim et al., 2014). Interestingly, the cardioprotective effects of intramyocardial administered CDC exosomes were maintained even when the exosomes were given 3 weeks after infarction (Ibrahim et al., 2014). MiR-146a was found to be enriched in CDC exosomes and was present at high levels in the infarcted heart following exosome injection and was associated with downregulation of interleukin-1 receptor associated kinase 1 (Irak1) and tumor necrosis factor receptor-associated factor 6 (Traf6), two signaling mediators of the toll-like receptr (TLR)-NF-kB axis. Oxidative stress was also suppressed by the downregulation of NADPH oxidase 4 (NOX-4) by miR-146a. Apart from miR-146a, other miRNAs such as miR-22 and miR-24 were found to play a key role in reducing myocardial injury (Ibrahim et al., 2014).
In a recent study, Couto et al. (2017) have demonstrated that CDC-derived exosomes may confer their cardioprotective effect by modulating macrophage polarization. Exosomes were administered into the LV cavity 20 min after reperfusion in a rat AMI model and via intramyocardial injection into the peri-infarct zone 30 min after reperfusion in a pig AMI model. Using rat and pig models of AMI, they demonstrated that exosomal transfer of miR-181b from CDCs into macrophages reduced protein kinase C-delta (PKC-δ) transcript levels and switched macrophages from a pro- to anti-inflammatory phenotype, demonstrating that cardioprotection elicited by exosomes administered after reperfusion may be mediated away from the heart and target circulating inflammatory cells. In another study, it was shown that CDC-derived exosomes contain the non-coding RNA, Y RNA fragment (EV-YF1), and this was transferred to macrophages and increased expression of IL-10 and mediated cardioprotection in a rat AMI model (Cambier et al., 2017). In terms of clinical translation, allogeneic CDCs generated from endomyocardial biopsies obtained from patients within 30 days of AMI and administered to the patient as a 24 hour infusion have been shown in the small CADUCEUS Phase 1 trial (Makkar et al., 2012) to reduce scar size on cardiovascular magnetic resonance scans compared to standard treatment, although no sham placebo was included in this trial. The Phase 2 ALLSTAR trial is currently underway to confirm the cardioprotective effects of CDCs (NCT01458405) (Chakravarty et al., 2017). Because this approach requires the generation of allogenic CDCs from myocardial tissue, it cannot be administered at the time of reperfusion to AMI patients, restricting its clinical application to the chronic LV remodeling period.
Stem-cell derived EVs as mediators of long-term cardioprotection
Stem cell-derived EVs have been shown to mediate beneficial effects on a number of processes that can prevent adverse post-infarct LV remodeling including pro-angiogenic effects, and immunomodulatory effects. Teng et al. (2015) have shown exosomes produced from bone marrow-derived MSCs have pro-angiogenic effects in terms of enhancing tube formation of human umbilical vein endothelial cells, and intramyocardial injection of exosomes into the infarct zone, increased the density of new functional capillaries, improved myocardial blood flow, limited MI size, and preserved cardiac function in a rat AMI model.
In previous sections, the acute cardioprotective effects of a single bolus of stem cell-derived EVs at the time of reperfusion have been highlighted. However, in order to confer ongoing cardioprotection to prevent adverse post-infarct LV remodeling one might consider repeat dosing or sustained delivery of EVs to the infarcted heart over the post-infarct period but this may of course be logistically challenging in AMI patients. Kang et al. (2015) demonstrated that exosomes produced from bone-marrow derived MSCs engineered to overexpress C-X-C chemokine receptor 4 (CXCR4) could be used to pretreat a cell-patch containing MSCs to enhance their cardioreparative effects, following implantation of the patch onto the infarcted heart after permanent LAD ligation. This resulted in a reduction in MI size, increased angiogenesis in the infarct zone, and improved cardiac function 4 weeks post-surgery. These cardioprotective effects were associated with upregulation of the PI3K/Akt pathway and inhibition of caspase 3 activity (Kang et al., 2015). Although this approach cannot be applied to AMI patients at the time of PPCI, it could potentially be applied to AMI patients needing open-chest cardiac bypass surgery.
In a pig AMI model, repeat daily dosing of MSC secretome over 7 days has been shown to have cardioprotective effects in terms of increased angiogenesis and less adverse LV remodeling, although in this study the cardioprotective effects of EVs were not studied (Timmers et al., 2011). Therefore, there remains a need for a technique to provide sustained delivery of stem cell secretome containing cardioreparative EVs to the infarcted heart over the first few weeks post-MI. In this regard, Kompa et al (2020), have used an innovative approach to achieve this, in which W8B2+ cardiac stem cells (CSC, generated from human atrial tissue) were encapsulated within a TheraCyte device, which was subcutaneously implanted into the rat dorsum immediately following permanent LAD ligation in order to provide sustained delivery of stem secretome release including EVs in the post-infarct period. After 4 weeks, angiogenesis was increased, and cardiomyocyte hypertrophy and interstitial fibrosis were attenuated, and cardiac function was preserved (Kompa et al., 2020). Crucially, it was shown that encapsulated CSCs were still viable after 4 weeks of implantation, and CSC-derived EVs were taken up by cardiomyocytes and endothelial cells within the infarcted heart (Kompa et al., 2020). Interestingly, a previous study has reported the clinical feasibility and safety of encapsulated allogeneic parathyroid tissue implantation in non-immunosuppressed patients using the TheraCyte device (Tibell et al., 2001). In that clinical study, encapsulated allogeneic cells survived up to 1 year post-transplantation, demonstrating the safety, feasibility, and efficacy of this device in non-immunosuppressed patients. Similar outcomes were observed in non-human primates (non-immunosuppressed) implanted with TheraCyte devices containing allogeneic fibroblasts (Tarantal et al., 2009).
Targeting EVs to the infarcted heart
The previous sections have highlighted the benefits of stem cell-derived EV-based therapy to reduce MI size and prevent adverse post-infarct LV remodeling, but there remain challenges to translate this therapeutic approach to the clinical setting for patient benefit. These include issues with bioavailability of EVs and potential off-target effects if using non-cardiac targeting EVs delivered systemically by the intravenous route as a cardioprotective strategy. Studies have shown that EVs delivered systemically by the intravenous route are rapidly cleared by macrophages and accumulate in the spleen, liver, and lungs (Mentkowski and Lang, 2019). Furthermore, an experimental study in pigs has shown that intramyocardial but not intracoronary delivery of CDC-derived EVs were effective at reducing MI and prevented adverse post-infarct LV remodeling (Gallet et al., 2017). However, intramyocardial injection of exosomes is invasive and challenging as a cardioprotective strategy in AMI patients. Studies have shown potential off-target effects of exosomes derived from bone marrow MSCs in terms of tumor progression (Roccaro et al., 2013). Therefore, the ability to target the delivery of EVs to the ischemic heart offers the opportunity to improve bioavailability and their cardioprotective efficacy, and avoid potential off-target effects, especially if EVs are to be delivered systemically by the intravenous route.
In this regard, recent studies have used cardiac homing or targeting peptides to target intravenously administered EVs to the ischemic heart following MI (Wiklander et al., 2015; Kim et al., 2018; Vandergriff et al., 2018; Wang et al., 2018; Mentkowski and Lang, 2019). Vangergriff et al. (2018) conjugated the cardiac-homing peptide, CSTSMLKAC (Kanki et al., 2011), to exosomes, and showed that the uptake of cardiac-homing exosomes was increased in neonatal rat cardiomyocytes, and the rat heart (in vivo) following acute myocardial IRI, and this was associated with less cell death, reduced MI size, and less adverse post-infarct LV remodeling. It has been shown that engineering cells to express the exosomal membrane protein, Lamp2b, fused with the neuron-specific RVG (rabies virus glycoprotein) peptide, enabled targeted delivery of siRNA to the brain using systemically administered exosomes (Alvarez-Erviti et al., 2011). Using a similar approach, HEK293 (Kim et al., 2018), MSCs (Wang et al., 2018), or CDCs (Mentkowski and Lang, 2019) have been engineered to produce exosomes that express cardiac-targeting peptides (APWHLSSQYSRT (Zahid et al., 2010), CSTSMLKAC (Kanki et al., 2011), and WLSEAGPVVTVRALRGTGSW (McGuire et al., 2004)) fused with Lamp2b on the exosomal membrane. These studies have shown uptake of the cardiac-targeting exosomes by H9c2 rat myoblasts was increased, when compared to non-cardiac targeting exosomes. In vivo studies in mice, have demonstrated that uptake by the heart of these cardiac-targeting exosomes was increased following acute myocardial IRI, and the cardiac-targeting exosomes reduced myocardial fibrosis, preserved LV function, attenuated myocardial inflammation (gene expression of interleukin-6, TNF-α, and interleukin-1β), decreased apoptotic cell death, and improved myocardial vascularity, when compared to non-cardiac targeting exosomes (Wang et al., 2018; Mentkowski and Lang, 2019).
Other approaches have been investigated to target exosomes to the infarcted heart. CXCL12 (also known as stromal cell-derived factor-1α), which is a member of the CXC chemokine family, which is overexpressed in ischemic tissues, is known to mediate cardiac repair following infarction by acting as a potent chemoattractant for CXCR4 expressing cells, including circulating progenitor cells. It has been reported that MSCs engineered to produce exosomes overexpressing CXCR4 reduced myocardial injury and prevented adverse post-infarct LV remodeling when applied as a sheet to infarcted heart tissue in a mouse model of acute IRI, to a greater extent than sheets of control MSCs (Kang et al., 2015). Cuillo et al (2019) have recently shown that overexpressing CXCR4 in CPC-derived exosomes increased their myocardial uptake and cardioprotective efficacy in a rat model of acute myocardial IRI and adverse LV remodeling, when compared to control exosomes, confirming that exosomes expressing CXCR4 can target the infarcted heart. Finally, Zhang et al. (2020) modified exosomes to express adhesive molecules such as macrophage adhesion ligand 1 lymphocyte function-associated antigen-1/intercelular adhesion molecule-1 (Mac1/(LFA1)/ICAM-1) that mimic the interaction of monocytes to endothelium following acute myocardial IRI. Interestingly, these monocyte-modified exosomes demonstrated increased uptake in the mouse heart following acute myocardial IRI, smaller MI size, and less adverse post-infarct LV remodeling (Zhang et al., 2020).
In summary, EVs can be modified to target the infarcted heart using cardiac-homing/targeting peptides and other methods, thereby increasing cardioprotective efficacy and reducing the risk of off-targets effects. Large animal studies are needed to investigate whether cardiac homing/targeting EVs administered by either the intravenous or intracoronary route can reduce MI size and prevent adverse post-infarct LV remodeling, thereby obviating the need for intramyocardial injection (Gallet et al., 2017).
Potential confounders of EV-mediated cardioprotection
Cardiovascular risk factors and co-morbidities such as diabetes, aging, obesity, metabolic syndrome, and their medications are known to impact the susceptibility of the heart to acute myocardial IRI and the efficacy of cardioprotective interventions such as ischemic conditioning (Sivaraman et al., 2010; Whittington et al., 2012; Ferdinandy et al., 2014; Crisafulli et al., 2020; Penna et al., 2020). These confounding factors may impact on EV-mediated cardioprotection via two main mechanisms, the first being by modulating the abundance, composition, and function of EVs, and the second by affecting the susceptibility of the myocardium to EV-mediated cardioprotection.
An early study demonstrated that microparticles were increased in patients with metabolic syndrome, and have been shown to induce endothelial dysfunction in an in vitro HUVEC model, and an in vivo mouse model, effects which were associated with attenuated endothelial nitric oxide synthase (eNOS) levels (Agouni et al., 2008). Circulating EVs in diabetes have been shown to be altered in terms of concentration, their cargo, and their influence on monocytes (Freeman et al., 2018). It has been found that EV concentrations are higher in individuals with type 2 diabetes, which may be attributed to increased EV secretion driven by insulin resistance. These EVs have been found to be preferentially taken up by B cells and monocytes, upregulating anti-apoptotic proteins and pro-inflammatory cyotkines. This appears to confer a contradictory effect in terms of promoting inflammation while reducing apoptosis (Freeman et al., 2018). Wu et al. (2020) observed that EVs from patients with diabetes contained higher levels of VEGF-A, which promoted EC cell migration and angiogenesis. Various inflammatory proteins were also found to be upregulated in EVs from diabetic individuals such as CD40, which is known to activate platelets causing inflammation and atherosclerosis (Rizvi et al., 2008). Age is also a confounder of EV-mediated cardioprotection that needs to be addressed. Recent findings from a clinical study (Carrozzo et al., 2020) suggested an increase in endogenous plasma EVs in older patients after cardiopulmonary bypass, conferring cardioprotection, which is contrary to the prevailing literature that age might reduce the cardioprotective nature of these endogenous EVs. These plasma EVs were found to reduce cell death in cardiomyocytes exposed to acute IRI in vitro, via anti-apoptotic and pro-survival proteins found in the cargo (Carrozzo et al., 2020).
The confounding effects of diabetes on the cardioprotective efficacy of EVs has been investigated by Davidson et al. (2018), who tested the protective effects of exosomes obtained from diabetic and non-diabetic patients undergoing cardiac surgery, diabetic and non-diabetic rats, and HUVEC cells exposed to high-glucose and normal glucose levels. They found that diabetic exosomes were unable to reduce cell death in primary rat cardiomyocytes subjected to simulated IRI, when compared to non-diabetic exosomes (Davidson et al., 2018). The failure to confer cardioprotection was attributed to a failure to activate the cytoprotective Erk1/2 and HSP27 signaling pathway, but the mechanisms through which diabetes modifies exosomes so they are unable to activate this signaling cascade in cardiomyocytes was not investigated in this study (Davidson et al., 2018). Interestingly, in this study non-diabetic exosomes were able to protect diabetic rat cardiomyocytes from acute IRI (Davidson et al., 2018), suggesting that an EV-based cardioprotective strategy may be still effective in diabetic patients with AMI.
Most experimental studies demonstrating cardioprotection with stem cell EVs have used healthy juvenile animal AMI models, which do not recapitulate the typical AMI patients who have various co-morbidities and is on a variety of co-medications, which may confound the cardioprotective effects of EVs (Ferdinandy et al., 2014). As such, experimental studies using relevant aged and diseased animal models are needed to test whether confounding factors such as age, co-morbidities, and co-medications affect the cardioprotective efficacy of stem cell-derived EVs before transitioning to testing EVs in clinical trials. Finally, the presence of certain co-medications, which are normally given to patients at the time of AMI, such as ticagrelor, atorvastatin, nitrates, and morphine, which are known to be cardioprotective in themselves, may either provide added cardioprotection to EV-mediated cardioprotection, or else mask any beneficial cardioprotective effects of EVs (Ferdinandy et al., 2014).
Summary and future perspectives
Experimental studies have demonstrated that stem cell-derived EVs can reduce MI size and prevent adverse post-infarct LV remodeling when administered by intravenous injection at the clinically relevant time-point of reperfusion, in rodent AMI models, and in the clinically relevant pig AMI model. However, a number of challenges face the clinical translation of EV-based therapy for the benefit of AMI patients. A comprehensive understanding of the pharmacodynamics, pharmacokinetics, and biodistribution of exosomes in patients is needed to guide the dosing and ensure safety of EV therapy. Large-scale purification of GMP-quality EVs will be required for testing in patients (Lener et al., 2015). In terms of a stem source for EV production, MSCs have several advantages including uncomplicated isolation process from a wide range of available human tissues (including adipose tissue), and the large ex vivo expansion capacity. Furthermore, given that a number of clinical studies have demonstrated safety of MSCs in patients, this may suggest that administration of MSC-derived EVs would unlikely lead to adverse effects. However, their drawback is that they can only undergo a finite number of cell divisions before their growth is arrested and they senesce, and so there is a need to constantly derive new batches of MSCs, and this incurs increasing costs for derivation, testing, and validation. This obstacle may be overcome by using immortalized hESC-MSCs, which have been demonstrated to produce high-quality EVs that are cardioprotective in animal AMI models (Chen et al., 2011). The effect of age and co-morbidities (such as diabetes and metabolic syndrome) and their medications as potential confounders of cardioprotective efficacy of stem cell-derived EVs will need to be explored before transitioning to clinical trials (Ferdinandy et al., 2014). Another approach to overcome the challenges of mass producing EVs include generating extracellular vesicle mimetics, which can allow large-scale production of EVs for clinical application, and are created by the extrusion of live cells though a series of micrometer-sized membranes and filtration, or by the process of sonication (Gangadaran and Ahn, 2020).
In addition to their roles as mediators of cardioprotection, EVs have been investigated as delivery vehicles for therapeutics. In this regard, EVs have key advantages including nano-sized dimensions, low toxicity and immunogenicity, high-stability in circulation, biocompatibility, and cell permeability, positioning them as promising carriers for efficient drug delivery for cardioprotection (Gangadaran and Ahn, 2020). Therapeutics can be loaded into EVs using a variety of methods including incubation with the drug solution (the most commonly used method but suffers from low loading efficiency), electroporation (where electric pulses are used to transiently permeabilize the bilayer membrane of the EVs), sonication (where ultrasound is used to dysregulate and permeabilize the EV membrane), freeze-thawing (which forms transient pores in the membrane), saponification (to induce the formation of small pores within lipid membranes), and extrusion (Mehryab et al., 2020). Due to the complex composition of EVs, their undefined biological functions, and potential safety concerns, extracellular vesicle mimetics or synthetic EVs that simulate natural exosomes with respect to their structure and functionality, but have a controlled composition, are being developed (Gangadaran and Ahn, 2020). Although EVs have been used to target drug delivery of cancer therapies it has yet to be applied to the field of cardioprotection. As highlighted in the previous section, EVs can be modified to target the infarcted heart thereby increasing bioavailability of any therapeutic cargo and minimizing off-target effects, thereby making cardiac-homing/targeting EVs as potential drug delivery systems for cardioprotection.
Despite these challenges a number of clinical studies are already ongoing and investigating the therapeutic potential of stem cell-derived EVs in a number of non-cardiac conditions including bronchopulmonary dysplasia, dystrophic epidermolysis bullosa, dry eye, macular holes, pancreatic adenocarcinoma, and type 1 diabetes mellitus (Tan et al., 2020). The closest medical condition to AMI is an ongoing clinical Phase 2 study investigating the safety and neuroprotective efficacy of allogenic MSC EVs enriched with miR-124 intravenously administered to five patients with acute ischemic stroke (NCT03384433). To date there are no ongoing clinical studies testing the safety and cardioprotective efficacy of stem cell-derived EVs in AMI patients as a treatment strategy to prevent HF. In AMI patients, there is the opportunity to inject stem cell-derived EVs via the coronary artery to provide local delivery of EVs to the ischemic myocardium. More challenging will be the repeated delivery of EVs over the weeks following AMI to provide sustained cardioprotection against adverse post-infarct LV remodeling. In this regard, the ability to deliver the cardioprotective benefits of stem cell secretome (including EVs) over a sustained period by encapsulating the stem cells in a subcutaneous device (Kompa et al., 2020) may provide sustained cardioreparative effects post-infarction for the first 3 months post-AMI, the period during which most LV remodeling occurs.
In summary, stem cell-derived EVs have huge therapeutic potential to mediate cardioprotection in terms of reducing MI size and preventing HF, and can in addition, be used to deliver cardioprotective therapies, thereby offering a synergistic approach to cardioprotection, and providing an innovative therapeutic strategy for improving clinical outcomes following AMI.
Acknowledgement
Sang-Ging Ong is supported by National Institutes of Health grantR00 HL130416 and R01 HL148756. Sauri Hernandez-Resendiz is supported by the Singapore Ministry of Health’s National Medical Research Council under its Open Fund-Young Individual Research Grant (OF-YIRG)–[NMRC/OFYIRG/0078/2018]. Derek Hausenloy is supported by the British Heart Foundation (CS/14/3/31002), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
Conflict of Interest
The authors declare no conflict of interest.
References
Aishwarya Prakash1
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore.
Gustavo E. Crespo-Avilan1,2,3
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore. 3Department of Biochemistry, Medical Faculty, Justus Liebig-University, Giessen, Germany.
Sauri Hernandez-Resendiz1,2,4
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore. 4Tecnologico de Monterrey, Centro de Biotecnologia-FEMSA, Nuevo Leon, Mexico.
Sang-Ging Ong5,6
5Department of Pharmacology, University of Illinois College of Medicine, Chicago, Illinois, United States of America. 6Division of Cardiology, Department of Medicine, University of Illinois College of Medicine, Chicago, Illinois, United States of America.
Derek J. Hausenloy1,2,7,8,9
1Cardiovascular and Metabolic Disorder Programme, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore. 7Yong Loo Lin School of Medicine, National University Singapore, Singapore. 8The Hatter Cardiovascular Institute, Institute of Cardiovascular Science, University College London, UK. 9Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Corresponding author:
Derek J. Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg

In a new window | Download PPT
Figure 1: Extracellular vesicles and cardioprotection. Extracellular vesicles (EVs) are known to act a mediators of endogenous cardioprotection by mediating intercellular communication between cardiomyocytes and endothelial cells (ECs), fibroblasts, pericytes, and immune cells. In addition, plasma EVs have been shown to mediate the cardioprotection elicited by endogenous strategies such as ischemic preconditioning (IPC) or remote ischemic conditioning (RIC). Stem cell-derived EVs from mesenchymal stromal cells (MSC), cardiac progenitor cells (CPC), and cardiosphere derived cells (CDC) have been shown to reduce myocardial infarct size and to prevent adverse left ventricular remodeling in small and large animal infarct models through a variety of mechanisms including cytoprotection, anti-apoptotic effects, metabolic effects, immunomodulation, increasing autophagy, and enhancing angiogenesis. Stem cell-derived EVs can be used as drug delivery systems to deliver cardioprotective drugs to the ischemic heart following acute myocardial infarction. Finally, addition of cardiac homing/targeting peptides to stem cell-derived EVs can target them to the ischemic heart, thereby increasing bioavailability and cardioprotective efficacy.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8944 | 22 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA