International bi-monthly journal of cell signaling, tissue protection, and translational research.
A taste of Alzheimer’s disease
Bella Gonzales-Portillo1, Madeline Saft1, You Jeong Park1, Blaise Cozene1, Nadia Sadanandan1, Justin Cho1, Marcella Reale2, Cesar V Borlongan1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
- 2Department of Medical Oral and Biotechnological Sciences, University "G. d'Annunzio" of Chieti-Pescara, Via dei Vestini 31, 66100 Chieti Scalo, Chieti, Italy.
Abstract
Our knowledge of the pathology of Alzheimer’s disease (AD), a progressive neurodegenerative disease, has significantly progressed due to numerous scientific advances. However, current clinical assessments of AD lack the certainty of the diagnosis; AD diagnosis is only certain through post-mortem examinations. Due to insufficient assurance of AD clinical diagnosis, effective and cost-efficient methods to detect early and accurate AD related markers are necessary to provide early therapeutic treatment. Specifically, discovering a peripheral biomarker may present accurate, reactive, duplicable means of detecting the neurodegenerative disease prior to the onset of symptoms, possibly leading to earlier, more effective treatments for prospective patients. Stem cells derived from the salivary glands may not only act as early biomarkers of AD but may also be harvested for cell-based regenerative purposes. The saliva may present an alternative source for biomarkers and stem cell harvest by providing noninvasive collections, and possible cost-effective, comfortable screening for large populations. With the possibility of stem cells present in the saliva, as well as the salivary glands, these therapeutic cells are a possible candidate for AD peripheral biomarker and AD treatment. This review provides a critical analysis of the possible use of saliva to identify a circulatory biomarker to diagnose early cognitive impairment of AD. In addition to suggestions of the potential implementation of saliva or salivary glands-derived stem cells for therapeutic purposes for AD, the emerging field of gut microbiome as a key target for AD pathology and treatment is also discussed. Because AD pathology is associated with microbiota modifications, and the gut microbiome can mount anti-neuroinflammatory activity, modulating microbiome levels through dietary alteration or antibiotic treatments may serve as a potential effective AD treatment.
Abstract
Our knowledge of the pathology of Alzheimer’s disease (AD), a progressive neurodegenerative disease, has significantly progressed due to numerous scientific advances. However, current clinical assessments of AD lack the certainty of the diagnosis; AD diagnosis is only certain through post-mortem examinations. Due to insufficient assurance of AD clinical diagnosis, effective and cost-efficient methods to detect early and accurate AD related markers are necessary to provide early therapeutic treatment. Specifically, discovering a peripheral biomarker may present accurate, reactive, duplicable means of detecting the neurodegenerative disease prior to the onset of symptoms, possibly leading to earlier, more effective treatments for prospective patients. Stem cells derived from the salivary glands may not only act as early biomarkers of AD but may also be harvested for cell-based regenerative purposes. The saliva may present an alternative source for biomarkers and stem cell harvest by providing noninvasive collections, and possible cost-effective, comfortable screening for large populations. With the possibility of stem cells present in the saliva, as well as the salivary glands, these therapeutic cells are a possible candidate for AD peripheral biomarker and AD treatment. This review provides a critical analysis of the possible use of saliva to identify a circulatory biomarker to diagnose early cognitive impairment of AD. In addition to suggestions of the potential implementation of saliva or salivary glands-derived stem cells for therapeutic purposes for AD, the emerging field of gut microbiome as a key target for AD pathology and treatment is also discussed. Because AD pathology is associated with microbiota modifications, and the gut microbiome can mount anti-neuroinflammatory activity, modulating microbiome levels through dietary alteration or antibiotic treatments may serve as a potential effective AD treatment.
Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disease that is primarily characterized by memory and language, decision-making, attention, and positioning impairment (Kirova et. al., 2015). AD affects more than 40 million people worldwide and is the most prevalent form of dementia (Sloane et al., 2002; Ferri et al., 2005). AD begins with an asymptomatic phase where symptoms first begin to appear in a person’s mid-60s. During this stage, the brain damage begins without symptoms, but manifests with the formation of amyloid plaques consisting of amyloid fibrils of amyloid β (Aβ) peptide and neurofibrillary tangles (NFTs), and both senile plaques and NFTs cause neural degeneration and neuronal loss. The damage begins in the hippocampus and as other neurons die, other areas of the brain are affected as well.
The earliest sign of cognitive impairment in AD is memory deficit and as AD progresses, memory loss and other cognitive difficulties increase as well. At this early stage, a diagnosis of mild AD is made, and as the disease progresses to a moderate phase, damage occurs in areas of the brain that control language, reasoning, sensory processing, and conscious thinking. In severe AD, plaques and tangles spread throughout the brain that subsequently succumbs to significant atrophy (Jack et al., 1998). Although the clinical symptoms of AD are frequently diagnosed in old age, the degenerative process may begin many years before the onset of the symptoms.
Diagnosis of AD is based on a series of psychological tests to assess memory and thinking skills, laboratory tests to evaluate genes and disease-related proteins, and imaging procedures that may help to detect AD dementia and how far the disease has progressed. However, AD can only be diagnosed with complete certainty after death, when microscopic examination of the brain reveals all the characteristic plaques and tangles (McKhann et al., 2011). Even though clinical testing and criteria will continue to be used for the diagnosis of AD, the identification of an increasing number of biomarkers will increase the pathophysiological specificity and the precocity of the diagnosis of AD. Given the present number of different AD biomarkers, it is inevitable that different combinations of test results may provide better diagnosis (Jack et al., 2013). However, further studies are needed to establish the priority of biomarkers and to determine their diagnosis value and validity.
The current state of Alzheimer’s disease diagnosis
The severity of AD neurodegeneration ranges from the early diagnostic stages, where mild disruptions manifest in brain function, to the detrimental stages, where the individual is unable to perform normal daily activities independently (Parnetti et al., 2019). Throughout the years, scientific advances have revealed the pathology of AD. This neurodegenerative disease first emerges in the hippocampus during an individual’s mid-60s asymptomatically. During the asymptomatic phase, symptoms of neurological damages are undetectable, and neurons begin to disconnect from neighboring neurons, inducing cell death and neurodegeneration (Akiyama et al., 2000; Thal et al., 2002; Karran et al., 2011; Heneka et al., 2015; Selkoe et al., 2016). The damage proceeds to form amyloid plaques, which consist of amyloid fibrils that are formed from peptide amyloid β (Aβ), and neurofibrillary tangles (NFTs), which is paired with helical filaments of the microtubule-associated protein tau. The formation of Aβ peptide and NFT further causes neural degeneration and neuronal cell death. Because the damage emerges from the hippocampus, the first notable neurological consequence involves memory dysfunction (Small et al., 1999). Over time, as the damage spreads and neuronal death increases, other regions of the brain become affected, leading to other executive dysfunctions and shrinkage of the brain. Towards the late, severe stages of AD, the damage affects the majority of the brain, presenting marked atrophy in brain tissues (Braak et al., 1991; Serrano-Pozo et al., 2011).
As mentioned previously, memory deficit is the first symptom of AD-induced cognitive impairment due to the emergence of neuronal damage in the hippocampus. During this state of AD, a diagnosis of mild AD is conducted. Memory loss and other cognitive dysfunctions worsen as AD progresses, disrupting the daily life of AD patients. Social disruptions caused by cognitive deficits include space-time disorientation, changes in personality and behavior, and difficulty in completing normal daily activities. During the moderate stage, AD-induced neurological damage spreads to the cerebral cortex, developing language, reasoning, and sensory impairment. Examples of the developed impairments include difficulty in learning, recognition, and eating. Additionally, AD patients may experience hallucinations or paranoia, developing impulsive behavior (Braak et al., 2011). During the severe stages of AD, neurostructural degeneration and significant atrophy are evident due to the spread of amyloid plaques and NFTs in the brain (Jack et al., 2013). AD patients are unable to perform daily tasks independently, relying on others for their care (Nelson et al., 2011).
Currently, AD diagnosis relies on psychological assessments on patients, examining memory and cognitive skills. Other sources of examination, such as laboratory tests and imaging procedures, analyze and detect biomarkers, dementia, and the progression of AD. However, even with various clinical techniques to diagnose AD, present examinations do not provide complete certainty of the diagnosis. Microscopic examinations of plaques and NFTs throughout the brain are necessary to diagnose patients with complete certainty only after death (McKhann et al., 2011). Additionally, because of the early asymptomatic stage of AD, clinical symptoms do not appear until the brain or regions of the brain has suffered significant amounts of damage. Clinical examinations do not capture the first occurrence of degenerative symptoms of AD until years later. Rather, current tests only detect the result of the neurological consequences of AD. Finding a peripheral biomarker that accurately detects AD prior to the onset of clinical symptoms may identify potential victims, initiating early therapeutic measures that may result in more effective treatments.
Where are early signs of Alzheimer's disease found?
Identification of specific biological markers may provide the ability to identify the presence of AD before manifestation or in the early stages of disease progression. The utilization of extra-neuronal tissues assists in elucidation of the underlying pathophysiological mechanisms of AD, such as the metabolic changes of β-amyloid protein precursor (APP), adjustments of ionic homeostasis, various transduction systems, and oxidative metabolism (Swomley et al., 2014). Other than plasma and cerebrospinal fluid (CSF), tissues such as fibroblasts, platelets, lymphocytes, and buccal cells are used to explore AD associated mechanisms (Roher et al., 2009; Neumann et al., 2011; Clark et al., 2013; De la Monte et al., 2017). Researchers study variation in brain and body fluids to indicate the AD-associated asymptomatic phase and potentially diagnose the disease before it manifests (Jack et al., 2013; O’Bryant et al., 2014; De la Monte et al., 2017).
Brain alterations in atrophy, amyloid plaque, NFT formation, alteration of regional activity, microgliosis, oxidative stress, and inflammation are assessed to aid in the diagnosis during the pre-clinical phase of AD. Image contrast agents, alongside computerized tomography (CT), magnetic resonance imaging (MRI), functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) are used to investigate these neural-changes (Matchar et al., 2004). Single nucleotide polymorphisms (SNPs) also serve as an indication of latent AD (Erdoğan et al., 2014; Giri et al., 2016). Flux in the central nervous system (CNS) is easily sensed through biochemical changes in CSF and plasma. Fluid composed of biomarkers represented by the Aβ40, Aβ42, tau, and the phosphorylated forms of tau (p-tau), indicate the manifestation from mild cognitive impairment (MCI) to dementia (Fagan et al., 2007). The lumbar puncture, a risk-ridden invasive procedure, is required to obtain CSF, a beneficial fluid containing markers that aid in the diagnosis of AD. CSF withdrawal may induce stress, which upregulates cortisol secretion and may influence biochemical markers. Similarly, lumbar punctures are associated with pain, discomfort, and various other side effects (Evans, 1998). Due to these reasons, minimally invasive procedures to obtain serum and plasma are practiced more commonly in clinical routine. Some believe that the ratio of plasma Aβ40/Aβ42 is indicative of the switch from MCI to AD (Graff-Radford et al., 2007). The potential of plasma analysis is hindered by comorbid diseases and many other factors that may easily influence AD biomarkers.
A more realistic and accurate approach in the prognosis of AD is the identification of Aβ and tau proteins in the CSF and blood due to their increased sensitivity and specificity to the disease. Other biological fluids have also been investigated as a potential indicative source of AD. Urine has been a candidate due to the presence of tau-associated neuronal thread protein (AD7c-NTP) (Zhang et al., 2018). AD7c-NTP is prevalent in CSF and urine of AD patients. Research has associated elevated AD7c-NTP with late-life depression (LLD) (Zhang et al., 2018), a disease linked with, and commonly mistaken for the early stages of AD. Further research is imperative to discover significant AD-indicative biomarkers in urine before moving into clinical practice (Takata et al., 2008; An et al., 2015; Zhang et al., 2018). Detection of latent AD is crucial to prevent disease progression and provide insight into the development of an effective treatment.
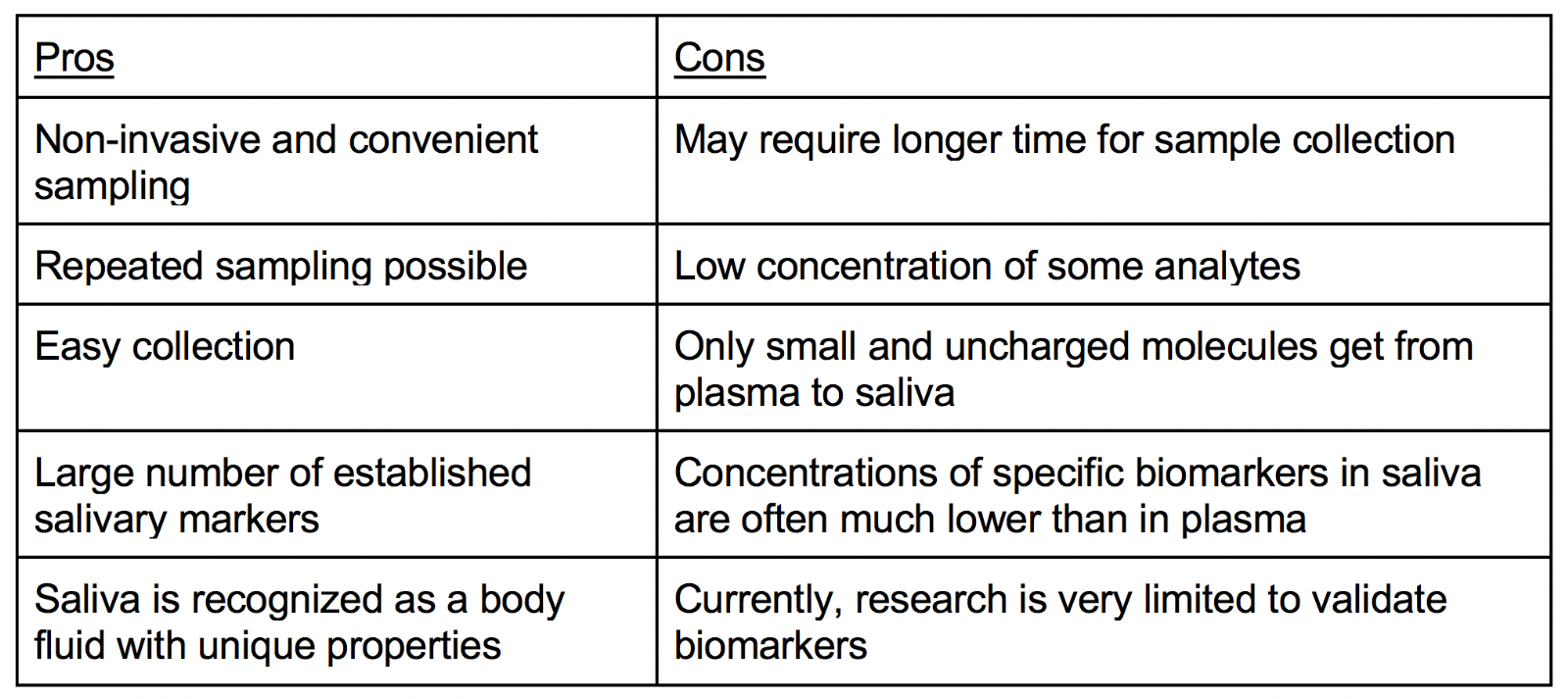
Saliva as a diagnostic tool
As a diagnostic tool, saliva offers many advantages because it can be collected noninvasively, offers a cost-effective approach, and is a readily available bodily fluid. Because saliva can be extracted without great risk of viral contamination in a fairly simple manner, it carries more therapeutic potential as a diagnostic tool than other physiological bodily fluids. There is minimal risk of contracting infections during saliva collection, which is advantageous, and saliva is beneficial for diagnostic use because it harbors a wide spectrum of proteins/peptides, nucleic acids, electrolytes, and hormones (Pfaffe et al., 2011). As a product of the salivary glands, saliva can be used to predict the onset of some diseases before their accumulation in vulnerable tissues causes clinical signs to appear (McGeer et al., 2020). Proteins from the CNS are excreted into the saliva and parts of the autonomic nervous system (ANS) are affected by AD including the brain stem, the hypothalamus, the cerebral neocortex, the insular cortex, and locus coeruleus. The main salivary glands in the mouth are the submandibular, the sublingual, and the parotid glands and they secrete saliva in response to cholinergic innervation from the glossopharyngeal cranial nerve and facial cranial nerve that are controlled by the ANS (Gleerup et al., 2019). Therefore, since some AD biomarkers, such as Aβ42 are expressed or produced in the salivary glands, a saliva sample can be used for AD diagnosis and treatment. Nonetheless, the lack of understanding regarding the biomolecules in saliva and their etiopathogenetic importance, as well as an insufficiency of high sensitive analysis networks, hinders the fully extensive implementation of saliva as a diagnostic tool. When compared to plasma, saliva presents itself to be a more effective diagnostic medium due to its non-invasive procedure and potential to survey wide-spread populations. However, the utilization of saliva does engender some difficulties. The concentration of biomolecules is lower in saliva than in serum. In addition, the chemical state of saliva is exceedingly variable because it swiftly alters its biochemical composition in response to a wide range of physiological conditions, stressors, stimuli, and injuries. Therefore, the biomolecules in saliva may be affected by the extraction procedure and diurnal/circadian discrepancies.
In order to distinguish the proteins in human saliva, the multi-faceted exploration of saliva’s various sources and components may be beneficial. The utilization of biomarkers in saliva for a multitude of maladies has recently progressed on account of proteomic studies, as well as immunoenzymatic assays, which aided in the qualitative and quantitative analysis of salivary proteins in physiological and pathological settings (Streckfus et al., 2002). The knowledge obtained from examinations of human saliva, encompassing genomics, epigenomics, transcriptomics, proteomics, metabolomics, enzyme-linked immunosorbent assays (ELISA), and quantitative real time polymerase chain reaction (qRT-PCR), has been coined as “salivaomics.” Indeed, procedures centered around mass spectrometry (MS) show significant efficacy. An amalgamation of the following methods have been employed to pinpoint salivary biomarkers in certain disorders: one- and two-dimensional gel electrophoresis, MS-rooted techniques, electro-spray ionization (ESI), liquid chromatography (LC), and 1H NMR (Streckfus et al., 2002). A microelectromechanical network-based fluid nanosensor test can potentially generate the ultrasensitive and ultra-specific identification of protein and RNA biomarkers in saliva. Presently, toxicology, endocrinology, drug testing, forensics, infectious diseases, HIV infections, and hormonal analyses all utilize saliva assessment.
Additionally, stem cells that develop prematurely and become dysfunctional with an age-related illness have been detected in salivary glands (Pringle et al., 2019). In neurodegenerative diseases with anomalous aging, such as AD, these stem cells’ susceptibility to age, along with β-amyloid plaque buildup in hyperphosphorylated and acetylated tau, can potentially generate the identification of rouge apoptotic cells (Fleitas et al., 2018; Janczura et al., 2018). Importantly, even with a decreased number of stem cells, aged salivary gland derived stem cells demonstrate greater stemness than those extracted from young salivary glands (Maimets et al., 2015). Moreover, salivary glands stand as a potent therapeutic source for stem cell-mediated treatment of age-associated diseases.
Indeed, there are very few biomarkers in nervous system disorders that warrant a non-invasive procedure and can be acquired with ease. The correlation between AD and damage to the salivary gland spurred by AD, specifically aberrant salivary volume have been investigated (Ship et al., 1990; Shi et al., 2011). AD salivary epithelial cells manifest modulated APPs, which spurs the emergence and build-up of neurotoxic Aβ peptides (Oh et al., 2006). Saliva is composed of electrolytes, peptides, proteins, glycoproteins, metabolites, lipids, RNA, micro RNA and DNA, predominantly found in the salivary glands. The submandibular and parotid glands, which are types of salivary glands regulated by the glossopharyngeal and facial nerve via parasympathetic innervation, manufacture a majority of the biomolecules found in saliva. Saliva fabrication begins in the acinar cells. Then, ductal cells adjust saliva and through passive and active transport, they move saliva to the oral cavity. The most utilized extracellular pathway is ultrafiltration, which takes place via tight junctions between cells. Because salivary glands are encompassed by capillaries, there are multiple components of saliva that do not arise from the salivary glands. These components include microbial products, gingival crevicular fluid, mucosal exudate, epithelial cells, and immune cells. Through either passive intracellular transport, active transport, or extracellular ultrafiltration, several elements found in plasma and cerebrospinal fluid are transferred to the saliva from blood. To enter saliva, biomolecules have to traverse the capillary wall, interstitial space, the basal cell membrane or cytoplasm of acinus and duct cells, as well as the luminal membrane. Furthermore, the molecules in blood match the compounds present in saliva, indicating that the biochemical makeup of saliva may hint at blood composition and the health of the body. Consequently, on account of the correlation between salivary glands and the nervous system, saliva may be an effective source of biomarkers for physiological and pathological settings of the neurological system.
In accordance with these findings, the utilization of saliva as a potential therapeutic target in assessing disease processes and observing efficacy of treatment has been further developed. Saliva-based tests are non-invasive, retrieved with ease, and require small amounts along with showing significant potential for frequent sampling and evaluation. In addition, they demonstrate great patient compliance, most likely from home for a certain length of time, altogether indicating its high practicality and feasibility. Saliva’s many therapeutic benefits suggest its efficacy as a diagnostic biomarker.
Table 1. Analysis of biomarkers in saliva and other body fluids.
Saliva collection
Evidently, non-saliva fluids, such as cerebrospinal fluid (CSF) and plasma, have displayed significant efficacy as therapeutic biomarkers. With respect to AD, therapeutic intervention at an early stage displays greatest curative efficiency, allowing for optimization of patient care and formulation of AD-modifying medications (Blennow et al., 2010; Bjerke et al., 2018). Therefore, biomarkers that can aid in the diagnosis of AD pre-symptomatically are of significant need (Blennow et al., 2010). Indeed, CSF and plasma have exhibited substantial potentiality as diagnostic tools for early detection of AD (Blennow et al., 2010). Recent preclinical and clinical studies have explored a multitude of biomolecules in these fluids that can serve AD biomarkers.
Over time, AD diagnostic markers in the CSF such as, total tau (t-Tau), phosphorylated tau (p-Tau), and Aβ42, have been well-established as potent contenders for the early detection of cognitive decline (Blennow et al., 2010; Molinuevo et al., 2018; Bjerke et al., 2018). These biomarkers have also been utilized as diagnostics in AD related diseases, such as mixed pathologies, atypical AD manifestations, and obscure clinical dementia (Bjerke et al., 2018). Investigations into additional CSF biomarkers have been recently conducted to bolster diagnostic efficacy of traditional biomarkers and to detect other pathological characteristics of AD, such as tau pathology, synaptic/neuronal degeneration, and amyloid mismetabolim (Bjerke et al., 2018). Notably, 139 out of 2327 identified proteins in the CSF appeared to be drastically modified in those with AD. Such proteins include microtubule associated protein tau (MAPT), neuronal pentraxin 2 (NPTX2), VGF, glial fibrillary acidic protein (GFAP), neural cell adhesion molecule 1 (NCAM1), protein kinase M (PKM), and tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein gamma (YWHAG) (Sathe et al., 2019). In addition, it was clinically elucidated that the progression of AD spurred the alteration of Aβ42 in CSF first, then Aβ42/Aβ40, P-tau, and T-tau. Following Aβ PET positivity, neurogranin, YKL-40, and neurofilament light were upregulated in the CSF (Palmqvist et al., 2019). In another clinical trial, the CSF from patients with mild cognitive impairment and more severe AD were compared. The presence of the neuronal pentraxin receptor-1 (NPTXR) most significantly distinguished the two disease stages, indicating its therapeutic potency as a diagnostic tool in AD progression (Begcevic et al., 2018). Interestingly, miRNAs demonstrate significant promise as a novel AD biomarker. A clinical trial examined 94 patients, who endured in-depth evaluation for dementia. As AD advanced, let-7b miRNA was upregulated, correlating with an increase in CSF CD4+ T cells, p-Tau, and t-Tau. Moreover, utilizing let-7b in conjunction with Aβ40-Aβ42 , t-tau, and p-tau, may further elevate their diagnostic potency in AD (Liu et al., 2018). Although certain CSF molecules are well-established as AD diagnostic markers, collection of CSF via diagnostic lumbar puncture is invasive and expensive (Doherty et al., 2014). Moreover, finding other sources of fluid biomarkers that warrant less invasive procedures and greater feasibility are crucial to establishing an effective diagnostic method for clinical detection of AD.
CSF levels of 5 neuroinflammation and cerebrovascular dysfunction biomarkers (YKL-40), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesions molecule 1 (VCAM-1), interleukin (IL)-15, and fms related receptor tyrosine kinase 1 (Flt-1)) increased during the preclinical and prodromal stages of AD (Janelidze et al., 2018). These 5 biomarkers correlated with CSF tau, predominantly in Aβ-positive individuals. Results also indicated increased concentrations of these biomarkers were associated with cortical thinning in the superior parietal cortex and precuneus (Janelidze et al., 2018). The data collected in this study also suggests that higher levels of neuroinflammatory and cerebrovascular biomarkers are correlated with cognitive decline and an increased risk of consequent development of AD. YKL-40 is a glycoprotein that is secreted in the CNS and is expressed via microglia and astrocytes (Canto et al., 2015; Querol-Vilaseca et al., 2017). YKL-40 is a potential biomarker of neuroinflammation in AD. Data indicates that there is an increased CSF level of YKL-40 in preclinical, prodromal, and dementia stages of AD (Alcolea et al., 2015; Craig-Schapiro et al., 2010; Janelidze et al., 2016; Mattsson et al., 2011). Other studies suggest that CSF YKL-40 is correlated with tau pathology, and has the potential of identifying individuals with abnormal tau levels in CSF (Alcolea et al., 2015; Baldacci et al., 2017).
Further investigation into other biological fluids that may serve as a potential diagnostic in AD has brought about the use of plasma biomarkers. Importantly, the biochemical states of the CSF and plasma often mirror each other in AD. For instance, blood Aβ level alternations reflect CSF AD biomarker changes and amyloid PET imaging (Nabers et al., 2018). As AD progressed in patients, plasma Aβ42, Aβ40, Aβ42/Aβ40, and p-Tau demonstrated similar AD-induced modifications to those same biomolecules in the CSF (Palmqvist et al., 2019). In patients with mild cognitive impairment and dementia due to AD, alterations in plasma neurofilament light (NFL) paralleled CSF NFL changes. Cognitive decline, atrophy induced by AD, and brain hypometabolism could be linked to high NFL levels in the plasma (( Mattsson et al., 2017 )). In addition, neuronal pentraxin 1 (NP1), originating in the CNS, may serve as a potent plasma biomarker, indicating synaptic neurodegeneration induced by soluble Aβ accumulation. Patients with mild cognitive impairment demonstrated upregulated levels of plasma NP1, and levels increased even more in patients in the early phase of AD (Ma et al., 2018). Indeed, mice models of AD have demonstrated that brain angiotensin-(1-7) (Ang(1-7)) levels are diminished in AD and can be linked to exacerbated tau pathology. Plasma levels of Ang-(1-7) mirror the changes in the brain, as plasma Ang-(1-7) is significantly lowered in AD patients. Notably, AD patients and controls could be differentiated based on plasma Ang-(1-7) concentrations (Jiang et al., 2016). Nonetheless, even with plasma’s numerous diagnostic benefits, it has its limitations, such that it is mildly invasive and may be difficult to apply to immense populations.
Although CSF and plasma have demonstrated efficacy as AD diagnostic fluids, saliva bears greater therapeutic potential in clinical practice due to its non-invasive and inexpensive collection procedure, as well as the plethora of biomolecules it holds. Saliva collection is not only highly feasible but also involves lower risk of infections (Pfaffe et al., 2011). Compared to both CSF and plasma, it is much less invasive and may screen more extensive populations. Importantly, the biomolecular composition of saliva often mirrors the biochemical conditions of both the CSF and plasma due to ultrafiltration, diffusion, and active transport (Gleerup et al., 2019). Potential contenders for salivary diagnostic biomarkers include Aβ42, tau, and lactoferrin (Gleerup et al., 2019). Notably, CSF reduction of Aβ42 in AD patients can be correlated with Aβ42 upregulation in saliva (Lee et al., 2017; McGeer et al., 2018). Nevertheless, further investigation into salivary AD biomarkers and saliva’s connection to the CSF and plasma is warranted in order to establish a clinically effective and practical diagnostic agent for AD.
Saliva collection standards
Saliva plays a big role in aiding digestion, protecting the oral cavity, and protecting teeth (Kubala et al., 2018). Due to this, the contents of saliva are always changing to better maintain homeostasis. When collecting saliva, many steps should be taken to ensure optimal quality upon collection. Between 9:00 and 11:00 is the best time to collect saliva due to its peak of physiochemical stability. All medications that affect salivary secretion should be halted a day prior to collection. Furthermore, food ingestion should be stopped 90 minutes before collection, and the mouth should be thoroughly rinsed with deionized water. Once saliva is collected, tests should be completed immediately to ensure accurate recording of the chemical and/or microbe concentrations tested for. If testing immediately is not an option, freezing saliva in glycerol at -80°C is the next course of action to provide the most accurate data (Kubala et al., 2018).
Saliva and AD: emerging results
Aβ42, known to aggregate most readily and be directly involved in the formation of neuritic plaques, has been detected in the saliva (Lee et al., 2017). Studies analyzing saliva samples from AD patients found higher levels of Aβ42 than those from controls when using ELISA-type tests (Lee et al., 2017). A similar finding was reported that using ELISA-type assays, which demonstrated significantly elevated levels of Aβ42 in the saliva of AD patients when compared with controls (Sabbagh et al., 2018). Photon-counting assessments were also used to assess the levels of Aβ40 and Aβ42 peptides using photo-multiplier tube in normal subjects and in patients with AD or MCI (Kim et al., 2014). There were higher levels of Aβ40 and Aβ42 in the saliva of AD patients compared with MCI and control groups, although differences in Aβ40 did not reach statistical significance.
Some saliva studies have reported elevated tau species, another protein implicated in the progression of AD. High sensitivity mass spectrometry analysis found no detectable levels of Aβ42 peptides but found elevated levels of salivary tau (Shi et al., 2011). Specifically, higher p-tau/t-tau ratios were measured in AD patients than in controls. In the CSF, T-tau and p-tau are elevated in AD patients and p-tau181 is the most studied phosphorylation site, which is used to identify AD (Blennow et al., 2011). Western blot analysis of saliva showed that one phosphorylation site, S396, p-tau/t-tau ratio was significantly higher in AD patients compared to age-matched control (Pekeles et al., 2018). Although the source of tau in the saliva has not yet been elucidated, the salivary glands are innervated and tau species have been detected in the submandibular gland (Dugger et al., 2016). Like Aβ peptides, tau species and salivary p-tau/t-tau ratios should be further investigated as potential biomarkers for AD.
Relationships between brain infections and AD and the role of antimicrobial peptides in AD pathology have been proposed (Welling et al., 2015). Lactoferrin is an antimicrobial glycoprotein that plays a role in immune response and inflammation and is present in secretory fluids including saliva. Decreased levels of salivary lactoferrin were found in amnestic MCI and AD patients when compared to control groups. Furthermore, lactoferrin levels were correlated with disease severity, mini-mental state examination scores, apolipoprotein E ε4 mutation (APOEε4) allele status, and t-tau in CSF in AD patients (Carro et al., 2017).
Cholinergic dysfunction is often seen in AD patients. Because of the salivary glands’ cholinergic innervation, their assessment may provide insight on alterations of cholinergic activity. Cholinergic activity has been evaluated in the CSF and in the serum of AD patients, with discordant results. Some studies have observed changes in acetylcholinesterase (AChE) activity in the serum (García-Ayllón et al., 2010) or CSF (Johansson et al., 2013), while other studies have not (Sirvio & Riekkinen, 1992). In salivary samples obtained from subjects meeting the criteria for ‘‘probable’’ AD, according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, and from sex- and age-matched controls, AChE activity was detected significantly lower in AD subjects (Sayer et al., 2004). In addition, there were significant differences in AChE activity between patients who responded to AChE-inhibitory (AChEI) therapy or not, which suggest AChE activity stands as a useful marker of central cholinergic activity (Sayer et al., 2004).
Advancements in metabolomics have also introduced another way to characterize saliva for diagnostic purposes. By analyzing the saliva metabolome with NMR, researchers have hypothesized differences in metabolomics can shed light on neurodegenerative disorders like AD. One study analyzed saliva metabolites from patients with AD, MCI, and controls using 1H NMR metabolomics (Yilmaz et al., 2017). There were 22 metabolites with concentrations that were significantly different in the saliva of MCI and AD patients compared to controls. Another study analyzed saliva from dementia patients with either AD or vascular dementia (Figueira et al., 2016). Dementia patients showed higher concentrations of acetic acid, histamine and propionate, and lower concentrations of dimethyl sulfone, glycerol, taurine, and succinate (Shan et al., 2015). Noteworthy metabolites include taurine, which has also been found significantly elevated in post-mortem brain tissue from AD patients (Graham et al., 2014). Taurine and hypotaurine metabolism dysregulation has been implicated in the pathophysiology of AD (Graham et al., 2014). Since taurine has a neuroprotective role in preventing neurotoxicity of Aβ it might have a potential role as biomarker for AD. Alterations in the histaminergic system have also been reported in AD, making histamine a candidate to further explore. Finally dimethyl sulfone and propionate are sensitive to diet and bacterial flora and may approximate the oral health of dementia patients. The pre-diagnostic value of the salivary metabolomics remains speculative and warrants further investigation with greater sample sizes. Since both p-Tau and Aβ42 are detectable in the saliva of patients with probable AD, these tools may be useful in monitoring the progression of the disease (Santos et al., 2017).
One of the first symptoms seen in early stages of AD is metabolic decline. This has been detected using fluoro-deoxyglucose positron emission tomography (FDG-PET) in patients with MCI (Ding et al., 2013). MCI patients display dysfunctional metabolism of lysine, lipids, citric acid cycle, and mitochondrial ketone bodies (Pagani et al., 2017). In patients with AD, metabolic disturbances in multiple networks including neurotransmission, lipid metabolism, and inflammation were detected in CSF and plasma (Han et al., 2011; Trushina et al., 2013). Such metabolomics analyses represent an attractive route to evaluate AD biomarker-cognition associations. Metabolic disturbances can reflect pre-symptomatic and subtle disease-related changes in the CNS. Saliva samples from AD patients and age-matched healthy controls have been used to identify metabolic changes (Liang et al., 2015). Significant differences in sphinganine- 1-phosphate, ornithine, phenyllactic acid, inosine, 3-dehydrocarnitine, and hypoxanthine were found between AD and control subjects. The most important metabolite for the predictive model of AD was upregulated sphinganine-1-phosphate. Altered sphingolipid metabolism has been detected in the brains of AD patients (Haughey et al., 2010), suggesting the possibility of using salivary metabolomics to diagnose AD. Metabolite biomarker panels were tailored for the discrimination between cognitively normal, cognitively impaired, MCI, and AD patients (Sapkota et al., 2018). The combination of complementary metabolomic platforms is a common strategy for the identification of biomarkers and disease diagnosis. In this study, methylguanosine, histidinyl-Phenylalanine, choline-cytidine were used to discriminate AD from control; amino-dihydroxybenzene, glucosylgalactosyl hydroxylysine-H2O, aminobutyric acid + H2 were used to discriminate AD from MCI, and; glucosylgalactosyl hydroxylysine-H2O, glutamine-carinitine were used to discriminate MCI from control. These results offer perspectives for potential use of non-invasive metabolite panels for the early diagnosis of AD (Sapkota et al., 2018).
The human salivary proteome has been shown to express 3,000 different proteins and peptides. The salivary proteome has been characterized in several neurologic and psychiatric diseases (Wormwood et al., 2015). AD is frequently associated with Down’s syndrome and increased levels of S100A7 and S100A12 detected in whole saliva of Down’s syndrome subjects may be a candidate biomarker for early onset of AD (Cabras et al., 2013). Beyond proteomics and metabolomics, genomic molecules like miRNA has also generated novel applications. About 1–4% genes in the human genome encode for miRNAs and a single miRNA can regulate as many as 200 mRNAs. These miRNAs are active in the cell in which they are produced, but can target mRNAs in a remote cell through endocrine signaling. miRNAs are present in CSF, plasma, urine, tears, and saliva (Weber et al., 2010). Recent studies have revealed circulating miRNAs in the CSF and serum of patients with AD, suggesting their use in the diagnosis of AD, either alone or in combination with other AD biomarkers (Cogswell et al., 2008; Alexandrov et al., 2012; Geekiyanage et al., 2012; Bekris et al. 2013). About 20 circulating miRNAs are significantly up-regulated, while 32 miRNAs are down-regulated in AD patients compared to control subjects (Kumar et al., 2013; Wu et al., 2016; Zendjabil et al., 2018). Although studies have explored the use of salivary miRNA as biomarkers for aging (Machida et al., 2015) and nervous system disease (Jin et al., 2013), there is limited knowledge on AD-associated miRNAs in human saliva. A high-throughput approach, such as miRNA next-generation sequencing, may provide more insight on disease-specific miRNA-profiles.
Finally, inflammation is associated with many neurodegenerative diseases including AD. The inflammatory response in the brain is characterized by activated glial cells that release pro-inflammatory cytokines, reactive oxygen species, and nitric oxide, altogether increasing Aβ synthesis and tau hyperphosphorylation, and subsequently contributing to neuronal cell death (Regen et al.; 2017). Peripheral inflammatory markers are able to cross the blood-brain barrier making peripheral cytokines potentially useful biomarkers to detect the progression of AD (Reale et al., 2012; Reale et al., 2014; Lai et al., 2017). Salivary IL-6, IL-1ß, tumor necrosis factor- alpha (TNF-α), matrix metalloproteinases (MMPs), and chemokines have been found to be associated with stress or local inflammatory conditions such as periodontitis, diseases of the salivary glands, and oral cancer. However, the relationship between salivary and blood cytokine levels has yet to be explored in AD.
Due to its accessibility, biomarkers obtained from saliva are gaining traction as a diagnostic tool for detecting diseases in early stages or tracking disease progression. The research in salivary biomarkers for AD is still in its nascent stage. However, advancements in isolation technology and fields of metabolomics, proteomics, and genomics may help clinicians expand their palates of diagnostic tools that may allow early detection and management of AD.
The potential of stem cells in the saliva to enhance diagnostic tool and treatment of AD
Age-sensitive stem cells reside in the salivary glands, and may provide a link to age-related AD (Kawakami et al., 2016). Salivary gland-originating stem cell exhaustion may be an indication of AD, and warrant saliva as a potential fluid for analysis. This non-invasive method attenuates all risks associated with CSP extraction. Along with using aged-cells as biomarkers, healthy salivary gland-derived stem-cells may be used as an AD transplantation therapy via treating age-induced salivary gland dysfunction.
Research uncovered that the co-culture of mouse early embryonic stem cells with human salivary gland-derived fibroblasts led to the differentiation of mouse early embryonic stem cells into human salivary gland-derived fibroblasts (Kawakami et al., 2013). Notably, these cells did not only differentiate and express salivary-gland related markers, but also were able to be transplanted with neogenetic ability (Kawakami et al., 2013). Furthermore, reduced acini concentration can indicate aging salivary glands (Maimets et al., 2016). The rejuvenation of salivary glands may be induced through treatment with Wnt-conditioned cells (Maimets et al., 2016).
Nerve growth factor (NGF), has demonstrated the ability to prevent cholinergic neuronal death and enhance memory, leading to the belief that it may prove to be an effective treatment for AD. Although NGF is not able to cross the blood-brain barrier (Borlongan et al., 2012), coupling with stem cells may intensify its neuroprotective effects. Human neural stem cells transfected with cholinergic acetyltransferase, stem cell line HB1.F3.ChAT, have shown promising results in mitigating AD related symptoms upon transplantation in experimental models. This may also be an effective treatment in other neurological conditions such as Parkinson’s disease (PD), stroke, and cancer (Borlongan et al., 2012). HB1.F3.ChAT cells possess the ability to differentiate into glial cells and neurons, while delivering specific genes such as ChAT. These findings also coincide with augmented acetylcholine concentrations and improved cognitive function (Borlongan et al., 2012). Alongside these appealing findings, grafted HB1.F3.ChAT cells can spread across the brain, which is imperative in combatting AD-associated widespread cholinergic cell loss. Further investigation in ameliorating detrimental host immune response and stem cell-related tumorigenesis must be completed before moving to clinical trials (Borlongan et al., 2012). Identification of aged and dysfunctional salivary gland stem cells, and the appropriate co-treatment with HB1.F3.ChAT cells and healthy salivary-gland derived stem cells may enhance the regenerative capacity of stem cell treatment within AD.
Saliva assay for early diagnosis of AD
Saliva functions as a viable and efficient diagnostic marker due to its fast and non-invasive features combined with high-performance analytical technologies. This sampling method poses no risks to the health of the patient. Biomolecules that exist within the saliva are either transferred through blood capillaries into the saliva or synthesized in salivary glands. Through using new technologies, we have the potential to identify genes, mRNA, proteins, and metabolites. This information would allow for the determination of biological markers present in saliva. Although further research needs to be conducted, saliva serves as an alternative to blood and tissue-based diagnostics for early detection of AD. Patients with AD also have the potential to receive customized treatments tailored to their distinct saliva biomarkers.
Saliva and stem cell therapies
As saliva has emerged as a potential sample source for the detection of disease biomarkers, salivary biomarkers also show promise in monitoring neurodegenerative and neuropsychiatric disorders such as autism, AD, PD, multiple sclerosis (MS) and Huntington’s disease (HD) (Farah et al., 2018). Analytical tests on saliva samples from MS patients were conducted to investigate saliva as a potential oxidative stress marker that could be used in disease monitoring (Karlik et al., 2015). Advanced Oxidation Protein Products (AOPP), Thiobarbituric Acid Reacting Substances (TBARS), and advanced glycation end products (AGEs), and fructosamine were the four stress markers tested (Karlik et al., 2015). AOPP is a marker for proper oxidation, TBARS is a marker of lipoperoxidation, and finally AGEs and fructosamine are markers of carbonyl stress. From these four stress markers, TBARS and AGEs were elevated in the saliva of MS patients compared to healthy individuals. AOPP remained the same in both healthy individuals and MS patients (Karlik et al., 2015).
Saliva is a highly accessible body fluid that contains proteins from the CNS (Spielmann et al., 2011). The brain stem, hypothalamus, cerebral neocortex, insular cortex, and locus coeruleus are all part of the , that can be affected by AD (Gleerup et al., 2019). AD can also degenerate nerve terminals in the cholinergic system, which controls the ANS and cardiovascular system. This degeneration is present in the preclinical phase of AD (Femminella et al., 2014). The submandibular, sublingual, and parotid glands produce saliva to react to cholinergic innervation, which is controlled by ANS. This modification in the ANS, which is seen in AD, may modify the production and composition of the saliva. An altered saliva composition may depict pathological changes in the CNS (Farah et al., 2018).
Stem cell therapy is also a viable approach in the treatment of AD. In rodent AD models, mesenchymal stem cell (MSC) transplantation was discovered to inhibit Aβ and tau related cell death (Zilka et al., 2011; Lee et al., 2012), minimize Aβ deposits and plaque formation (Kim et al., 2013; Yang et al., 2013; Yun et al., 2013; Naaldijk et al., 2016), and recover spatial learning and memory loss (Lee et al., 2012; Kim et al., 2013; Yang et al., 2013; Yun et al., 2013). It was also found to trigger neurogenesis, synaptogenesis, and neuronal differentiation (Zilka et al., 2011; Yang et al., 2013; Oh et al., 2015). MSCs that are administered to patients intravenously also can cross the blood-brain barrier and migrate to areas of neuronal injury without eliciting an immune response (Ra et al., 2011). Preclinical studies indicate that stem cells are a viable treatment option for AD, however further research and human trials need to be conducted (Duncan et al., 2017).
Gut microbiome and AD
Dietary patterns have an impact on the function of the salivary and gut microbiota. By examining the diversity, composition, and functional potential of the salivary microbiota in vegans and omnivores using gene amplicon sequencing, the effects of a long-term diet can be discovered. Results indicate that long-term dietary patterns and distinct nutrients assist in shaping the salivary microbiota (Han et al., 2011).
Evidence suggests that gut microbiota interacts with AD pathogenesis by disrupting neuroinflammation and metabolic homeostasis (Seo et al., 2020). Since AD has been associated with gut microbial alterations, gut bacterial communities may serve as a target for therapeutic intervention (Vogt et al., 2017). While there are currently no preventative measures or treatments for AD, pathogenic microbes play a significant role in the development of AD pathology and also the use of dietary measures and antibiotics.
AD is closely related to an imbalance of the gut microbiota. Modulation of gut microbiota through personalized diet or the intervention of beneficial microbiota could alter microbial partners and their products, including amyloid protein and serve as a potential treatment for AD (Sochocka et al., 2019). Modifications of the gut microbiota induced by oral bacteriotherapy in animal studies show changes in genes involved in inflammatory and neural plasticity processes that positively impact neural functions. Increased plasma concentration of gut hormones such as ghrelin, leptin, glucagon-like peptide-1 (GLP1), and gastric inhibitory polypeptide (GIP) demonstrate improvement in cognitive function. Additionally, animals treated with a probiotic mixture showed higher plasma levels of such hormones and demonstrated that modification of microbiota can produce anti-inflammatory effects (Sochocka et al., 2019).
Modulating the gut microbiome through dietary means serves as an effective therapeutic approach to reduce inflammation associated with AD. While few studies have evaluated dietary patterns and gut microbiota, there exist positive associations between the Mediterranean diet (MD) and an increased number of beneficial microbiota species that have anti-inflammatory effects (McGrattan et al., 2019). Adherence to the MD diet has been associated with better cognitive performance and reduced risk of cognitive impairment. The DASH diet, commonly used in patients with hypertension, produces similar findings and both diets may protect against neurodegeneration in AD patients (McGrattan et al., 2019). Further research is needed to understand the relationship between the gut microbiome and cognitive health and the effects of dietary measures on alterations in the gut microbiota.
Recent studies have demonstrated a link between microbiota perturbation on AD pathology with different manipulation strategies including the treatment of antibiotics. The treatment of an amyloid mouse model with antibiotics altered the composition of the gut microbiota and reduced Aβ deposition in the hippocampus and multiple cortical areas. Using antibiotic treatment, altering the gut microbiota in AD models caused changes in neuroinflammation and Aβ deposition likely due to regulation of pro-inflammatory T helper 1 cells (Th1) (Wang et al., 2019). Abnormal alteration of gut microbiota during AD development, along with Aβ deposition and tau phosphorylation, elevates levels of amino acids, specifically isoleucine and phenylalanine, exacerbating Th1 cell production through metabolic disorders. The peripheral pro-inflammatory cells circulate into the brain and crosstalk with neuronal M1 microglial cells, inducing neuroinflammation and cognitive impairment and accelerating AD symptoms. However, administering antibiotics can normalize gut microbiota by targeting disordered metabolites. Resurrecting normal gut microbiota activity and reducing Aβ deposition relieve metabolic disorders, reducing Th1 cell circulation into the brain and neuroinflammatory activity. By altering gut microbiota to regulate Th1 cell infiltration into the brain, antibiotics may serve as a potent AD treatment. However, further investigations are needed to characterize the gut microbiota to other diverse aspects of AD pathology such as tauopathy and neurodegeneration (Seo et al., 2020). Testing in AD patients supports the novel idea to reduce pathological bacteria and increase beneficial bacteria with probiotics as a potential therapy. The ability to manipulate the gut microbiota serves as a promising strategy to prevent AD pathogenesis. Additionally, nutrients and other dietary compounds can also influence neuroinflammatory processes and diets rich in anti-inflammatory components attenuate neuroinflammation within the brain and indirectly from the gut microbiome (McGrattan et al., 2019).
Conclusion
The time and money required to obtain the multidisciplinary clinical diagnosis of AD prevent many patients from receiving an adequate diagnosis until the disease has already evolved and treatments are no longer effective in later stages. The development of new therapies has focused on rapid and cost-effective screening of patients for AD. The presence in saliva of biomarkers that correlate with disease pathology and symptomatology provides a safe method to assess patients and provide personalized treatment regimes. Saliva-based assays provide alternatives to methods such as neuroimaging, volumetric changes of the brain and assessment of Aβ42, total tau, and p-tau in CSF. Genetic testing serves as another diagnostic tool for AD, however, it does not provide the ability to differentiate between different forms of dementia and is very costly to use regularly.
Salivary diagnostic tests demonstrate great clinical potential due to the fact that they serve as a non-invasive and inexpensive diagnostic tool for AD patients. The ability of salivary diagnostic tests to identify and understand saliva-based biomarkers could help establish saliva as a diagnostic bio-fluid. However, research on saliva biomarker testing is still limited to date and conflicting results are possibly due to different methods of collecting the salivary sample, its preservation, and their specificity and sensitivity that are found not to be comparable. In order to produce accurate identification of salivary biomarkers, proper collection and saliva processing protocols must be standardized.
The recognition that AD pathology may entail a key peripheral component, namely the contribution of gut microbiome, warrants investigation of this tissue source not just as a pathologic tool but also as a target for AD treatment. The role of food diet and the potential of stem cells to alter the gut microbiome present as novel avenues of investigations for AD pathology and its treatment. With the entry of conditioning the gut microbiome towards therapeutic purposes against AD equate to potent conditioning medicine-based treatment and management of the disease.
In conclusion, reviewed literature has shown that there are potent biomarkers available in saliva, but more research is required to conduct large-scale human studies to identify and validate these biomarkers for early detection and management of AD. Equally compelling evidence suggests innovative approaches exploring gut microbiome and stem cell therapy, which may provide new diagnostic and treatment strategies for AD.
References
Bella Gonzales-Portillo1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Madeline Saft1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
You Jeong Park1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Blaise Cozene1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Nadia Sadanandan1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Justin Cho1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Marcella Reale2
2Department of Medical Oral and Biotechnological Sciences, University "G. d'Annunzio" of Chieti-Pescara, Via dei Vestini 31, 66100 Chieti Scalo, Chieti, Italy.
Cesar V Borlongan1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Blaise Cozene and Nadia Sadanandan contributed equally to this article.
Corresponding author:
Cesar V Borlongan
Email: cborlong@usf.edu
Table 1. Analysis of biomarkers in saliva and other body fluids.

Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8402 | 22 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA