Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The potential of umbilical cord to afford natural conditioning in three different mesenchymal stem cell populations for stroke therapy
Time:2020-09-17
Number:9075
Nadia Sadanandan1, Madeline Saft1, You Jeong Park1, Justin Cho1, Cesar V. Borlongan1, Jea-Young Lee1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Conditioning Medicine 2020. 3(4): 199-203.
Abstract
Stroke is a damaging illness with high mortality rates and limited therapeutic options. Stem-cell mediated regenerative therapy has risen as a novel approach for stroke treatment. However, utilizing stem cells with high post-transplantation cellular viability is critical to maximize therapeutic efficacy. Stroke leads to oxygen and glucose deprivation in the brain. Therefore, stem cells that are transplanted must be resistant to hypoxic conditions. During development, the human umbilical cord (hUC) is exposed to hypoxia, and the stem cells derived from the hUC are therefore subjected to ischemic conditions. These stem cells’ ability to survive in such harsh environments suggests that they may display robust viability after transplantation into similarly damaged tissues, such as in the ischemic brain. Evidence regarding this novel feature of umbilical cord stem cells indicates that they may serve as graftable stem cells. Moreover, the natural conditioning of umbilical cord stem cells to oxygen-deprived environments may engender hypoxia/ischemia-resistant characteristics in these cells, serving as an effective therapeutic tool in Conditioning Medicine.
Abstract
Stroke is a damaging illness with high mortality rates and limited therapeutic options. Stem-cell mediated regenerative therapy has risen as a novel approach for stroke treatment. However, utilizing stem cells with high post-transplantation cellular viability is critical to maximize therapeutic efficacy. Stroke leads to oxygen and glucose deprivation in the brain. Therefore, stem cells that are transplanted must be resistant to hypoxic conditions. During development, the human umbilical cord (hUC) is exposed to hypoxia, and the stem cells derived from the hUC are therefore subjected to ischemic conditions. These stem cells’ ability to survive in such harsh environments suggests that they may display robust viability after transplantation into similarly damaged tissues, such as in the ischemic brain. Evidence regarding this novel feature of umbilical cord stem cells indicates that they may serve as graftable stem cells. Moreover, the natural conditioning of umbilical cord stem cells to oxygen-deprived environments may engender hypoxia/ischemia-resistant characteristics in these cells, serving as an effective therapeutic tool in Conditioning Medicine.
Stem-cell mediated mitochondrial repair in ischemic stroke
Stroke is currently the fifth leading cause of death in America and causes disabling neurological deficits like cognitive impairment, hemiparesis, sensory disturbance, and aphasia (Ovbiagele, 2013). By 2030, 3.88% of the American population over the age of 18 are predicted to have had a stroke and total annual stroke-related costs will reach $240.67 billion (Ovbiagele, 2013). Mitochondrial dysfunction plays a key role in the pathological progression of stroke (Yang, 2018). Mitochondria are double membrane-bound organelles containing electron transport proteins, the adenosine triphosphate (ATP) synthetase complex, and ATP/adenosine diphosphate (ADP) transport proteins. During stroke, insufficient blood circulation leads to oxygen and glucose deprivation (OGD). This disruption prevents the mitochondria from performing oxidative phosphorylation, a process that generates 92% of the overall cellular ATP, and cells are no longer able to maintain their metabolic functions (Tarasov, 2012; Yang, 2018; Heyck, 2019). The defective oxidative metabolism leads to excess production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) following the re-establishment of blood flow during reperfusion (Yang, 2018). The oxidative stress overwhelms the neutralization capacity of the endogenous antioxidant system, and the overproduction of ROS and RNS ultimately results in cell death through the destruction of proteins, lipids, and DNA (Yang, 2018).
Stem cell-based therapies aim to replace dead cells and protect injured tissue by supplementing endogenous repair mechanisms or providing trophic support, reducing the inflammatory response (Stonesifer, 2017; Russo, 2018a). Recently, stem cells have demonstrated the ability to transfer healthy mitochondria into damaged cells via tunneling nanotubes (TNTs), gap junctions, microvesicles, cell fusion, and uptake of mitochondria (Russo, 2018b). While the signaling mechanism that initiates mitochondrial transfer is unknown, the phenomenon is most likely involved in the restoration of host cell function observed after stem cell transplantation. Mitochondrial transfer has been observed in several cell types including mesenchymal stem cells (MSCs), astrocytes, neurons, and endothelial progenitor cells (Paliwal, 2018; Borlongan, 2019). Human umbilical cord (hUC)-derived MSCs (hUC-MSCs) are multipotent, display self-renewal, and modulate the immune response through the release of cytokines (La Rocca, 2013; Caprnda, 2017; Borlongan, 2019). Because of the hUC’s natural hypoxic and glucose-poor environment, it is hypothesized to be an excellent cell-source for regenerative therapies against ischemic injury like stroke.
Ameliorating stroke-induced mitochondrial damage utilizing three different hUC-MSCS
Stem cell therapy, mainly using hUC-MSCs, has shown profound effects on stroke-induced injuries by reducing inflammatory response and transferring healthy mitochondria (Tajiri, 2014; Caprnda, 2017; Russo, 2018a). However, investigations into whether MSCs derived from different compartments of the hUC possess different levels of therapeutic effects have rarely been conducted (Davies, 2017).
Due to this lack of understanding, cell metabolic profile and mitochondrial function of hUC-MSCs were analyzed from three distinct compartments: Wharton’s jelly (WJ), perivascular region (PV), and cord lining (CL). All three variations of hUC-MSCs were known to possess immunomodulatory and anti-inflammatory abilities (La Rocca, 2013; Magatti, 2016; Caprnda, 2017). To compare the three cellular types of hUC-MSCs, each cell type was isolated and extracted using the most optimal technique to ensure the survival of the cells. The hUC-MSCs groups were each placed under normal and pathological stroke environments, and their performances under both conditions were assessed by utilizing cell energy phenotype and mito stress tests (Russo, 2020).
The findings generated enticing evidence regarding MSCs and their three different populations. Immunofluorescence staining revealed mesenchymal markers cluster of differenction (CD) 90 (CD90) and CD73 in all tested types of hUC-MSCs while also displaying additional distinct markers in each MSC population. Specifically, WJ-MSCs expressed the stemness marker octamer-binding transription factor 4 (OCT4), the endothelial cell adhesion molecular marker CD146 was expressed in PV-MSCs, and the monocyte/macrophage marker CD14 was expressed in CL-derived MSCs (Russo, 2020), thus each MSC population displayed key mediators of the inflammatory response in hypoxia. The XF Cell Energy Phenotype Test further elucidated that all three types of hUC-MSCs significantly increased the metabolic response. Additionally, the test revealed the dormant nature of the metabolic profile of each tested hUC-MSCs, providing evidence for the low levels of mitochondrial and glycolytic activities in MSCs (Hu, 2016). The cell mito stress test was used to analyze the mitochondrial function of each MSC derivative under ischemic environments. The test produced evidence of the hUC-MSCs’ ability to withstand ischemic conditions with PV-MSCs having the highest mitochondrial activity, which is likely due to hUC consisting of only two arteries and a vein, simulating a nutrient-deprived environment similar to ischemic conditions. The cell viability test demonstrated the survivability and adaptability of PV-, WJ-, and CL-MSCs post-OGD/reperfusion (OGD/R), providing additional support for hUC-MSCs ability to survive in ischemic/reperfused conditions. OGD/R environments were found to significantly alter PV-MSC metabolism, but not the viability. On the other hand, the metabolism and viability of both WJ-MSC and CL-MSC remained normal with CL-MSC being the least affected (Russo, 2020). Despite the difference in the compartments that were derived from, CL-, WJ-, and PV-MSCs, they are all potential sources of mitochondria for stem cell therapy for stroke.
Examining the efficacy of the three different hUC-MSCs in ischemic conditions
In order to determine the three hUC-MSCs’ potential, PV-, WJ-, and CL-MSCs were isolated from hUCs to determine their energy metabolism profile, mitochondrial function, and survival capacity in both normal and post ischemic/reperfused conditions. Methods such as measurement of mitochondrial mass, determination of mtDNA copy number, western blot analysis of glycolytic and mitochondrial enzymes, assay of the expression of mitochondrial biogenesis-associated genes, assessment of intracellular ATP content, and oxygen consumption rate (OCR) measurement using the 782 OxygenMeter, and quantization of radioactive labeled glucose have been previously performed (Hu, 2016). The Seahorse analyzer has also been used for OCR measurement in differentiated adipose-derived MSCs and induced pluripotent stem cells-derived mesenchymal progenitor cells. The SFR-Shake Flask Reader was also employed to analyze hUC-MSCs metabolism by measuring dissolved O2 and pH values in culture medium (Lavrentieva, 2010).
In particular, the Seahorse Analyzer that measures OCR and the extracellular acidification rate (ECAR) was used to determine the energy metabolism of the three hUC-MSC populations. The Seahorse Analyzer was used to perform the cell energy phenotype test and cell mito stress test. Results of the cell energy phenotype test demonstrated that all three hUC-MSCs share similar energy metabolism, exhibited a quiescent phenotype, and thus, maintained both mitochondrial and glycolytic activities at low levels. Under stress conditions, the three types of MSCs showed an increase in the glycolytic pathway to balance the reduction of mitochondrial respiration. Moreover, the cell mito stress test was performed in both normal and after OGD/R to analyze the mitochondrial activity of the three types of hUC-MSCs in ischemic/reperfused conditions. The cell viability test demonstrated that the three populations of hUC-MSCs adopted resistance and adapted to ischemic/reperfusion conditions. The ability of all three types of hUC-MSCs to survive in stroke conditions is suggested by the fact that the number of cells increased and they were not affected by OGD/R treatment. Moreover, since robust cell viability under ischemic conditions is crucial to effective stem-cell transplantation (Borlongan, 1998), hUC-MSCs show substantial therapeutic potential in stroke.
The isolation of hUC-MSCs for repairing the mitochondria has the potential to serve as a novel stroke therapy (Al Naem, 2019). Cell-based regenerative medicine may benefit from mitochondrial transplantation and serves as a promising method of treatment for many neurological disorders (Borlongan, 1996; Borlongan, 1999; Borlongan, 2008). The PV-, WJ-, and CL-MSCs have the ability to adapt to ischemic environments and all display similar energy metabolism and mitochondrial function. While PV-MSCs recorded the highest OCR values and CL-MSCs were least affected by OGD/R conditions, further investigations are needed to understand the basis behind these differences amongst the three types of hUC-MSCs. Nevertheless, hUC-MSCs display viable mitochondria after ischemic/reperfusion injury and serve as a promising cell source for mitochondria-based stem cell therapy in stroke.
Clinical evidence supporting the therapeutic potential of UC stem cells in stroke
Clinical trials investigating the safety and efficacy of UC stem cells in stroke therapy have been performed. In one such study, UC-MSCs were administered intra-arterially through a catheter proximal to the lesion area in three ischemic stroke patients and one hemorrhagic stroke patient. Importantly, intra-artery delivery showed no adverse effects. After six months, muscle vigor and Rankin scale score were ameliorated in two of the ischemic stroke patients. However, the hemorrhagic stroke patient did not show these improvements. Nonetheless, the administration of UC-MSCs intra-arterially demonstrates substantial potential as an ischemic stroke therapy, as it greatly improves neurological performance post-stroke (Jiang, 2013). Another clinical trial administered a combined cell therapy to chronic stroke patients using olfactory ensheathing cells, neural progenitor cells, UC-MSCs, and Schwann cells. The UC-MSCs were delivered via intrathecal implantation. The patients demonstrated improvement in neurological performance, displaying ameliorated speech patterns, muscle vigor and tension, balance, and breathing, as well as higher barthel index and clinical neurologic impairment scale scores (Chen, 2013). Moreover, stem cells derived from the UC show significant potency as a stroke therapeutic; however, further investigation into the safety and efficacy of these cells in pre-clinical studies is warranted.
Although UC stem cells demonstrate significant therapeutic potential in stroke treatment, some limitations must be overcome to ensure optimal clinical application. Limitations of UC stem cells in stroke therapy include their inadequate differentiative, migratory, and survival capabilities (Cao, 2020), as well as low cell numbers (Berglund, 2017). However, the chemical compound tetramethylpyrazine (TMP) has been shown to ameliorate some of these shortcomings. TMP enhanced the migratory abilities of UC-MSCs) in vitro and the improvements were dose dependent. Notably, the high dose of TMP significantly bolstered UC-MSC differentiation into cells demonstrating neuron-specific markers (Cao, 2020). Indeed, the area of the UC from which the stem cells are derived affects the cells’ therapeutic potential. Compared to fetal placenta-MSCs, CL-MSCs, and WJ-MSCs, cord-placenta junction (CPJ)-MSCs displayed greater differentiative and proliferative properties (Beeravolu, 2017). Utilizing CPJ-MSCs in stroke therapy may effectively ameliorate low-cell count and differential capacity. Furthermore, before prime clinical implementation for UC stem cells can be established, preclinical trials exploring ways to combat the limitations of these cells in stroke therapy is critical.
Pre-conditioning hUC-MSCs in an hypoxic environment
Since hUC-MSCs are exposed to sub-lethal hypoxic conditions early on, they build greater resistance to hypoxia and ischemia, making them potent therapeutic agents for stem-cell based stroke therapy. Understanding a stem cell’s physiological microenvironment is essential to enhancing the cell’s regenerative capabilities (Nekanti, 2010). Therefore, capitalizing on hUC-MSC’s developmental subjection to oxygen-deprivation via hypoxic preconditioning may further bolster hUC-MSC’s restorative effects in ischemic stroke.
Hypoxia preconditioning is a widely accepted practice in the cardiovascular and cerebrovascular systems and stems from the idea that tissue exposure to hypoxic or ischemic circumstances can bolster that tissue’s resilience to future oxidative stress (Li, 2017). Early sub-lethal subjection to hypoxia can impart significant neuroprotection and improve neuronal viability in ischemic stroke (Smeyne, 2019). For instance, astrocytes preconditioned with ischemia shielded neurons from OGD damage and substantially lowered cellular death (Narayanan, 2017). In addition, hypoxic pre-conditioning of bone marrow-derived MSCs spurred greater release of vascular endothelial growth factor (VEGF), enhancing neuronal survival in ischemic conditions (Zhang, 2019). Furthermore, hypoxic conditioning may be equally promising with hUC-MSCs for stroke therapy.
Notably, WJ-MSCs cultured under 3% oxygen conditions demonstrated greater proliferation and cell survival, as well as, improved mobilization when compared to WJ-MSCs cultured under 21% oxygen (Obradovic, 2019). Similar results were achieved in another 3% oxygen level hU-MSC culture, as hUC-MSC multiplication and differentiation into cardiomyocyte-like cells increased. The pre-conditioning also safeguarded hUC-MSCs from the deleterious effects of chemical hypoxia (Zhang, 2015). Indeed, hypoxic conditioning bolstered the expression of developmental mesodermal and endothelial genes in WJ-MSCs, indicating improved differential capabilities. WJ-MSCs also demonstrated escalated proliferation (Nekanti, 2010). Moreover, hypoxic preconditioning elevates hUC-MSCs’ differentiative and proliferative capabilities, accentuating their therapeutic potential for ameliorating ischemic stroke injury.
Additionally, hypoxic pre-conditioning may fortify hUC-MSCs’ curative abilities in ischemic stroke through mechanisms other than improved differentiation and proliferation, such as diminishing inflammatory factors and promoting autophagy. When hypoxia pre-conditioned hUC-MSCs were administered to ischemic hindlimb injury mice models, angiogenesis occurred at a greater rate than in mice treated with normoxic hUC-MSCs. Importantly, hypoxia pre-conditioned hUC-MSCs more effectively attenuated the expression of inflammatory factors, such as IL-1 and IL-20, compared to normoxic hUC-MSCS (Han, 2016). In addition, autophagy plays an important role in generating neuroprotection in ischemic stroke. After hUC-MSCs were cultivated in a hypobaric hypoxia chamber at a 1% oxygen level, autophagy became more prevalent, indicating the neuroprotective potential of hypoxic preconditioning (Yin, 2018). In sum, exposing hUC-MSCs to hypoxia prior to treatment may invigorate hUC-MSCs’ restorative effects in ischemic stroke therapy. However, further investigation exploring the efficacy of hypoxic preconditioning in hUC-MSC-mediated stroke treatment is warranted.
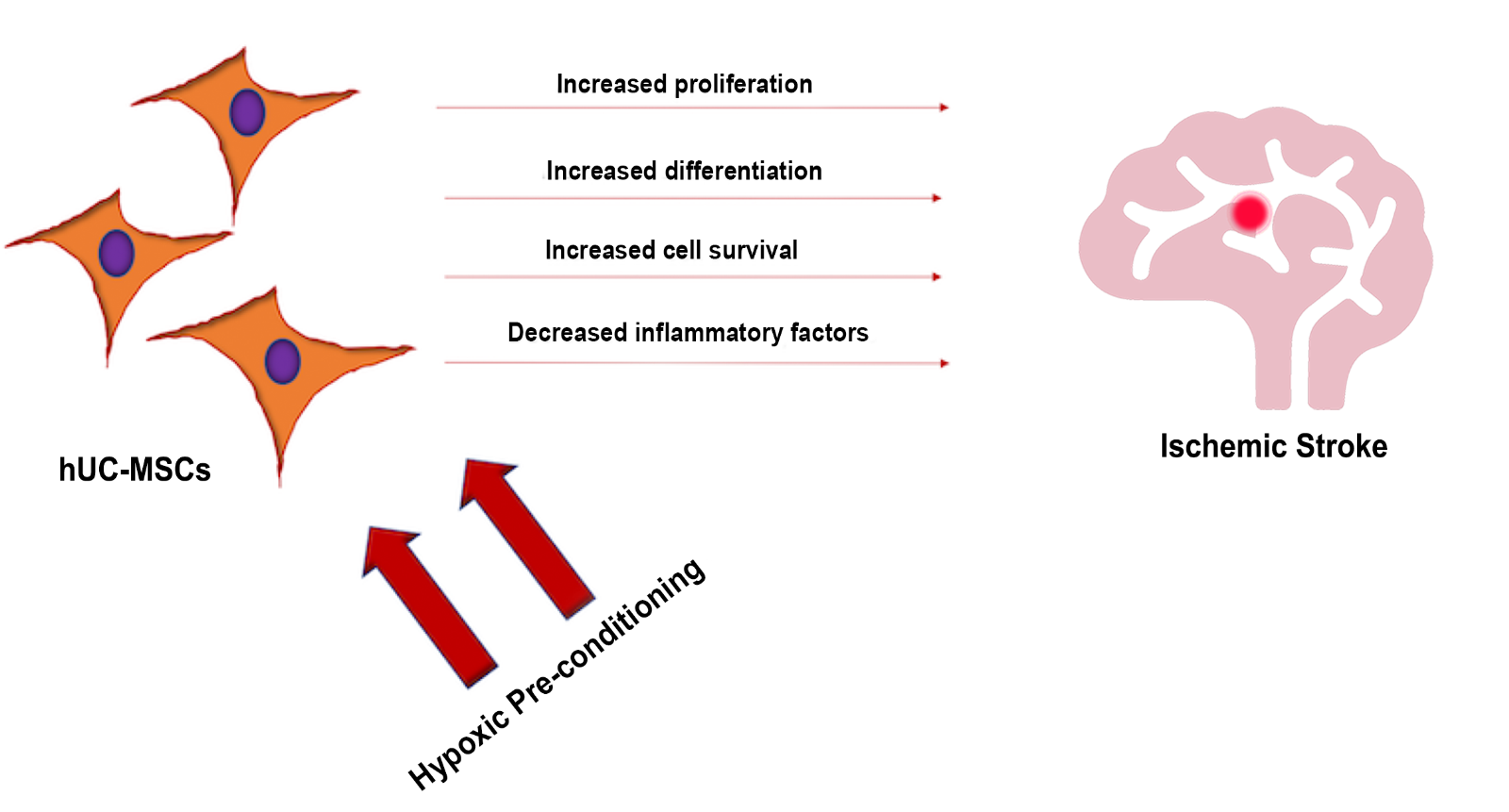
In a new window | Download PPT
Figure 1: The exposure of hUC-MSCs to hypoxic pre-conditioning may increase cell proliferation differentiation, and overall cell survival, as well as decrease inflammatory factors, altogether reducing the stroke-induced pathological deficits.
Conclusion
With limited therapeutic options, stem-cell therapy serves as a novel treatment for stroke. Since stroke pathology is characterized by hypoxic/ischemic conditions, viable stem cells must demonstrate resistance to such harmful environments. The use of hUC-MSCs serves as a promising stem cell source because the hUC is exposed to hypoxia during childbirth, allowing for hUC-derived stem cells to develop resistance against future ischemic conditions. The use of Seahorse technology demonstrates the ability of PV-, WJ-, and CL-MSCs to display substantial mitochondrial function and survival under ischemic/reperfusion conditions. Moreover, hUC-MSCs exemplify viability under hypoxic conditions and demonstrate potential to survive after transplantation to injured tissue, including the ischemic brain. In conclusion, conditioning hUC-MSCs to build resistance to hypoxic/ischemic settings stands as a potential therapeutic option for mitochondria-based stem cell therapy in stroke-induced ischemic injury.
References
Nadia Sadanandan1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Madeline Saft1*
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
You Jeong Park1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Justin Cho1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Cesar V. Borlongan1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Jea-Young Lee1
1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Nadia Sadanandan and Madeline Saft contributed equally to this article.
Corresponding author:
Cesar V. Borlongan
Email: cborlong@usf.edu

In a new window | Download PPT
Figure 1: The exposure of hUC-MSCs to hypoxic pre-conditioning may increase cell proliferation differentiation, and overall cell survival, as well as decrease inflammatory factors, altogether reducing the stroke-induced pathological deficits.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9075 | 3 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA