Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
The wondrous Warburg Effect and its implications in stem cell therapeutics
Time:2020-09-17
Number:6366
Blaise Cozene1, Bella Gonzales-Portillo1, Jeffrey Farooq1, Cesar V Borlongan1, Yuji Kaneko1
Author Affiliations


- 1Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Conditioning Medicine 2020. 3(4): 192-198.
Abstract
Rhynchophylline (Rhy) stimulates neurogenesis and interferes with the signaling pathways of various neurodegenerative diseases such as Alzheimer disease, Parkinson disease, epilepsy, and amyotrophic lateral sclerosis. A specific intracellular environment is required to maintain cell proliferation and to attain pluripotency. Pluripotency can be achieved through the specific expression of genes and proteins. This study evaluated whether Rhy promotes metabolic regulation in bone marrow human mesenchymal stromal cells (BM-hMSCs). Results indicate that Rhy modulates cellular activity by managing the N-methyl-D-aspartate receptor subunit and mitochondria, as well as levels of basic fibroblast growth factor, brain-derived neurotrophic factor, oxytocin receptor, and adenosine triphosphate. Rhy modifies the expression level of BM-MSC genes related to proliferation and differentiation. It was also observed that Rhy augments lactate dehydrogenase activity and the glycolytic flow ratio while decreasing pyruvate dehydrogenase activity. This indicates a BM-hMSC metabolic shift of mitochondrial oxidative phosphorylation into aerobic glycolysis, a metabolism similar to the Warburg effect. Uncovering the mechanism of action of Rhy-induced BM-hMSC modification allows for enhancement of cell transplantation via amplification of stem cell metabolic activity. Pre-treatment to induce a metabolic shift in stem cells may provide more favorable outcomes in cell-transplantation therapies. Here, we discuss Rhy-induced Warburg effect as a potential “conditioning” strategy for stem cell therapy.
Keywords: Pre-conditioning; conditioning medicine; Warburg Effect; cell-based therapy; stem cell metabolism
Abstract
Rhynchophylline (Rhy) stimulates neurogenesis and interferes with the signaling pathways of various neurodegenerative diseases such as Alzheimer disease, Parkinson disease, epilepsy, and amyotrophic lateral sclerosis. A specific intracellular environment is required to maintain cell proliferation and to attain pluripotency. Pluripotency can be achieved through the specific expression of genes and proteins. This study evaluated whether Rhy promotes metabolic regulation in bone marrow human mesenchymal stromal cells (BM-hMSCs). Results indicate that Rhy modulates cellular activity by managing the N-methyl-D-aspartate receptor subunit and mitochondria, as well as levels of basic fibroblast growth factor, brain-derived neurotrophic factor, oxytocin receptor, and adenosine triphosphate. Rhy modifies the expression level of BM-MSC genes related to proliferation and differentiation. It was also observed that Rhy augments lactate dehydrogenase activity and the glycolytic flow ratio while decreasing pyruvate dehydrogenase activity. This indicates a BM-hMSC metabolic shift of mitochondrial oxidative phosphorylation into aerobic glycolysis, a metabolism similar to the Warburg effect. Uncovering the mechanism of action of Rhy-induced BM-hMSC modification allows for enhancement of cell transplantation via amplification of stem cell metabolic activity. Pre-treatment to induce a metabolic shift in stem cells may provide more favorable outcomes in cell-transplantation therapies. Here, we discuss Rhy-induced Warburg effect as a potential “conditioning” strategy for stem cell therapy.
Keywords: Pre-conditioning; conditioning medicine; Warburg Effect; cell-based therapy; stem cell metabolism
A Glimpse of Warburg Effects
Mesenchymal stem cells (MSCs) have the ability to differentiate into cells of the mesoderm, endoderm, and ectoderm (Uccelli et al., 2008). MSCs are used to treat patients with myocardial infarction, neurodegenerative diseases, skeletal tissue injuries, and organ failure (Nombela-Arrieta et al., 2011). MSCs have the capacity to regenerate tissues due to their pleiotropic function, and do not express major histocompatibility complex II, making them an effective therapy. Under normoxic conditions, MSCs utilize both glycolysis and oxidative phosphorylation (OXPHOS) for adenosine triphosphate (ATP) production. Undifferentiated MSC metabolism during proliferation is associated with aerobic/anaerobic glycolysis (Ito and Suda, 2014; Pattappa et al., 2011). However, OXPHOS in the mitochondria is associated with differentiated MSCs and correlates with impaired stem cell multipotency (Ito and Suda, 2014). This indicates that the metabolism of proliferating MSCs depends on glycolytic metabolism with aerobic glycolysis rather than OXPHOS, a metabolism resonant with the Warburg Effect (Gatenby and Gillies, 2004).
Glycolysis normally occurs under anaerobic conditions, however the cell must continue respiration in either an aerobic or anaerobic environment, advancing the name “aerobic glycolysis.” Aerobic glycolysis refers to the conversion of glucose to lactate in the presence of oxygen (Dashty, 2013). This condition is also referred to as the Warburg Effect. The final stages of aerobic glycolysis produce extracellular lactic acid, although enough oxygen is present to allow OXPHOS to occur (Dashty, 2013).
The Warburg Effect has been thoroughly studied in cancer cells (Liberti and Locasale, 2016). Cancer cells alter their metabolism to expedite growth, increase glucose uptake, and hasten the fermentation of glucose to lactate. The Warburg Effect is also observed in pluripotent stem cells (Cha et al., 2017). Studies show that the metabolism of primed pluripotent stem cells are inclined toward glycolysis rather than OXPHOS, demonstrating a Warburg-like effect (Folmes et al., 2012; Rafalski et al., 2012; Zhang et al., 2012; Ito and Suda, 2014). Newer studies demonstrate that the metabolic shift from OXPHOS to glycolysis is essential for bioenergetics, biosynthetic capacity, and epigenetic regulation in human pluripotent stem cells (hPSCs) (Varum et al., 2011; Zhang et al., 2012; Zhou et al., 2012), which is also supported by metabolomics analyses (Folmes et al., 2011; Panopoulos et al., 2012; Moussaieff et al., 2015). Mouse embryonic stem cells exhibit a bivalent bioenergetics state able to switch from glycolysis to OXPHOS on demand (Zhou et al., 2012), indicating that stem cell metabolic bioenergetics does not follow rigid glycolytic or cell respiration pathways, but displays a Warburg Effect phenomenon.
N-methyl-D-aspartate receptor (NMDAR) and Rhynchophylline (Rhy)
A glutamate gated ion channel, the NMDAR, mediates physiological and pathological function in the central nervous system (Lai et al., 2014). Composed of seven subunits, the NMDAR is induced via allosteric modulation. The equilibrium shift of the NMDAR subunit expression pattern plays a pivotal role in the determination of the receptor diversity in the plasma membrane (Kaneko et al., 2018). Erythropoietin-producing hepatocellular A4 (EphA4) is a part of the A subclass of Eph receptor tyrosine kinases whose transduction pathways initiate receptor-expressing cells and ligand-expressing cells (Boyd et al., 2014). These signaling pathways are highly regulated and are often associated with neurodegenerative disorders (Boyd et al., 2014). The activation of EphA4 signaling pathways leads to dendritic spine loss as well as reduction of a-amino-3-hydroxy-5-methyl-4-isoxazo-lepropionic acid receptor expression (Fu et al., 2011). Together, these conditions act as potential mechanisms that underlie synaptic dysfunction in Alzheimer disease (AD) (Hsieh et al., 2006).
The alkaloid Rhy is derived from the extraction of Uncaria rhynchophylla and is a component of Kampo medicine known as Choto-san (Shellard and Lala, 1978). Rhy has the ability to travel across the blood brain barrier, form neuronal networks, upregulate neurogenesis, and function as a noncompetitive antagonist of the NMDAR (Kawakami et al., 2011; Antonchick et al., 2013). Rhy is capable of binding to EphA4 and postponing the EphA4-dependent signaling pathway (Fu et al., 2014). Targeting EphA4 with Rhy as a selective EphA4 inhibitor may provide favorable results in alleviating AD-induced effects, however further elucidation is necessary. Based on these novel functional properties of Rhy, it has also been advanced as a promising candidate drug for AD, Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and epilepsy (Fu et al., 2014; Hashimoto, 2015; Shao et al., 2016; Hu et al., 2018).
BM-hMSC’s and Rhy
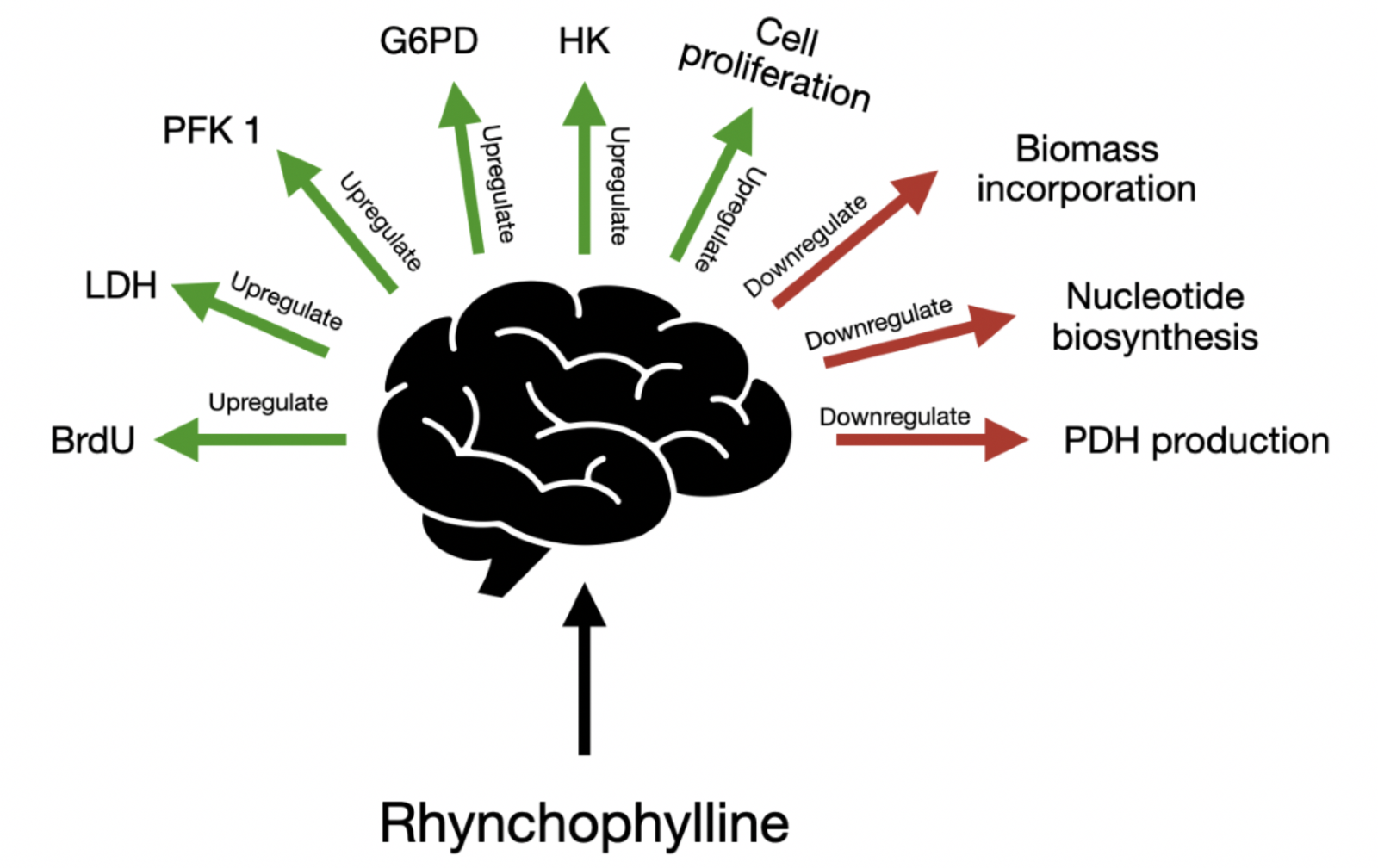
Rhy increases the proliferative abilities of BM-hMSC’s. Rhy also controls the mRNA expression level of transcription factors. Rhy triggers the proliferation of BM-hMSCs by controlling the glucose metabolic pathway. Rhy has the potential to upregulate bromodeoxyuridine (BrdU), lactate dehydrogenase (LDH), phosphofructokinase 1 (PFK1), glucose-6-phosphate dehydrogenase (G6PD), and hexokinase (HK), while downregulating biomass incorporation, nucleotide biosynthesis, and pyruvate dehydrogenase (PDH) production. Although Rhy modulates the glucose metabolic pathway, L-lactate levels, glucose uptake, and pyruvate kinase (PK) production are unaltered.
Rhy potently enhances BrdU activity (Kaneko et al., 2020), a nucleoside marker of the S-phase cell cycle that regulates mRNA and protein expression (Lagace et al., 2010; Aviner et al., 2015). Rhy-treatment of BM-hMSCs increases ATP content while reducing MTT and cytochrome c activity, as well as impairing total protein synthesis (Kaneko et al., 2020). OXPHOS occurs in the mitochondria and generates ATP through complete glucose catabolism to supply the energy cells need for replication. Rhy preferentially redistributes OXPHOS resources towards the tricarboxylic acid (TCA) cycle but does not directly alter mitochondrial function. Rhy also decreases the concentration of the proteins basic fibroblast growth factor (FGFβ), oxytocin receptor (OXTR), and brain-derived neurotrophic factor (BDNF), suppresses gene expression of kruppel-like factor 4 (Klf4), nanog, and collagen type 1 alpha 1 (Col1A), enhances expression of runt-related transcription factor 3 (Runx3) and peroxisome proliferator-activated receptor gamma (Pparγ), and does not alter expression of octamer-binding transcription factor 4 (Oct4), sex-determining region y-box 2 (Sox2), cellular myelocytomatosis (cMyc), and Runx2 (Kaneko et al., 2020). In addition, Rhy inhibits the activity of EphA4, a negative regulator of NMDAR, and increases the levels of the NMDA receptor subunit (GluN) 1, GluN2B, and GluN3A (Fu et al., 2014; Ito and Suda, 2014; Kaneko et al., 2020). Treatment of BM-hMSCs with Rhy increases glucose transporter 4 (GLUT4) expression and bolsters the activity of HK, G6PD, PFK1, and LDH while simultaneously impairing activity of PDH (Kaneko et al., 2020). Rhy-mediated regulation of each of these proteins and genes are discussed below as well as referenced in figure 1.
In a new window | Download PPT
Figure 1: Rhynchophylline affords its therapeutic effects through multiple mechanisms, including upregulation of BrdU, LDH, PFK1, G6PD, HK and cell proliferation, while downregulation biomass incorporation, nucleotide biosynthesis, and PDH production.
Behind the Scenes: Rhynchophylline and Stem Cells
Rhy enhances BM-hMSC proliferation, glycolysis, Runx3 and Pparγ expression, and synthesis of GluN1, GluN2B, and GluN3A proteins under constant mitochondrial membrane potential. Rhy simultaneously impairs mitochondrial activity, inhibits Col1A, Nanog, and Klf4 mRNA expression, and reduces OXTR, FGFβ, and BDNF protein production. Importantly, Rhy-induced modulation of cell growth and metabolism pathways potentially drives proliferation and enhances the stemness of BM-hMSCs. Rhy treatment effectively shifts MSC metabolism from OXPHOS to glycolysis, even in high oxygen environments (Kaneko et al., 2020). This suggests that Rhy exerts its beneficial effects on BM-hMSC growth by augmenting the Warburg Effect.
Rhy’s capacity to precisely modulate protein expression of several key targets enhances its potency as a therapeutic compound. FGFβ leverages RUNX2 expression to trigger chondrocyte and osteoblast maturation, while BDNF works cooperatively to modulate BM-hMSC expression of RUNX2 (Teplyuk et al., 2009; Chen et al., 2014; Feng et al., 2018). Further, OXTR promotes MSC differentiation to cardiomyogenic and osteogenic pathways (Elabd et al., 2008; Elabd et al., 2014). While Rhy enhances neuronal stem cell production of BDNF for axon elongation and development of synaptic connections (Antonchick et al., 2013), and downregulates the expression of all three aforementioned factors: FBFβ, BDNF, and OXTR in BM-hMSCs (Kaneko et al., 2020).
Rhy also alters the expression of BM-hMSC transcription factors Klf4, Col1A, and Nanog. KLF4 is a zinc-finger transcription factor that controls cell growth, differentiation, and death, and further serves as a modulator for reprogramming somatic cellular processes. KLF4 also supports homeostatic mechanisms by regulating expression of genes with GC/GT-rich promoters. RUNX2, through its α-1 core-binding factor subunit, promotes the development of osteoblasts and hypertrophic chondrocytes, as well as bone matrix-protein gene Col1A expression. Importantly, KLF4 inhibits RUNX2, leading to an imbalance between bone-forming osteoclast and bone-resorbing osteoclast activity that manifests as pathologic bone conditions, including periodontal disease, osteoporosis, Paget’s disease, and rheumatoid arthritis (Walsh et al., 2006). Despite downregulation of Klf4 and Col1A, Rhy does not impact Runx2 gene expression. Nanog influences stemness and terminal differentiation through its interaction with stemness genes Oct4 and Sox2. Downregulation of Nanog expression in stem cells creates an intermediary environment that tempers transitional barriers and thus, enhances cell differentiation. Rhy-treatment of BM-hMSCs markedly diminishes Nanog levels but does not alter Oct4 and Sox2 (Kaneko et al., 2020). This likely occurs because while decreased stem cell Nanog expression accelerates cellular differentiation, the quantity of Nanog-expressing cells does not affect the differentiative and proliferative abilities of BM-hMSCs (Pierantozzi et al., 2010).
Pparγ serves as a control center for adipogenesis and lipid metabolism, neuronal differentiation and neuronal stem cell growth, as well as angiogenesis and osteoclastogenesis (Tontonoz et al., 1994; Sun et al., 2013). Because adipogenesis largely depends on chromatin remodeling and creation of de novo enhancers, whereas osteoblastic differentiation is mainly upregulated by enhancer activation, undifferentiated BM-hMSCs preferentially differentiate into osteoblasts (Deb, 2019; Rauch et al., 2019). In this regard, Pparγ effectively blocks pre-osteoblastic differentiation via indirect inhibition of RUNX2 (Sun et al., 2013). RUNX3 is a tumor suppressor and is critical in differentiation of neuronal stem cells and production of neurons. Rhy determines the direction of BM-hMSC differentiation by enhancing the expression of both Runx3 and Ppary, while leaving protooncogene cMyc expression in BM-hMSCs unchanged (Kaneko et al., 2020).
The EphA4 signal transduction cascade is located upstream of the NMDAR and is linked to BM-hMSC expression of GluN1, GluN2B, and GluN3A. Rhy blocks EphA4-mediated cell signaling, and increases expression of GluN1, GluN2B, and GluN3A while not affecting that of EphA4, GluN2A, and GluN3B. The glycine-gate cation channel is composed of GluN1, GluN3A, and GluN3B subunits, while combining GluN1 and GluN2 to form GluN3 effectively reduces the channel (Traynelis et al., 2010; Kaneko et al., 2018). In addition, the combination of GluN1 and GluN3A subunits forms the NMDAR. BM-hMSC differentiation towards osteogenic and chondrogenic pathways requires NMDAR-mediated cell signaling (Yoneda, 2017). NMDAR signal transduction synergistically enhances RUNX2-facilitated gene expression and subsequent chondrocyte differentiation (Takarada et al., 2009). Rhy does not regulate expression of Runx2, however, it significantly reduces expression of Col1A. This occurs due to divergent cellular processes present in undifferentiated and differentiating cells that ultimately comprise the heterogenic and multipotent BM-hMSC progenitor cell population (Kaneko et al., 2020).
The maximum lifespan and differentiative capability of hematopoietic stem cells is largely governed by the significant effect of BM-hMSC aging on BM cell regulatory efficacy (Ito and Suda, 2014). Decreasing regenerative capabilities, reducing the quiescent phase, and downregulating stem cell reserves are all means by which the glycolytic pathway mitigates the harmful effects of aging stem cells (Ito and Suda, 2014). Undifferentiated hMSCs provide more ATP to glycolytic metabolism compared to their differentiated counterparts, due to increased expression of glycolytic enzymes and reduced expression of OXPHOS enzymes in the undifferentiated population. Enhanced glucose flux combined with higher amounts of key enzymes in glycolytic metabolism are needed to upregulate aerobic glycolysis. Alternatively, decreased levels of these enzymes shift cellular metabolism towards OXPHOS. Rhy modulates the activity of glycolytic enzymes and glucose and lactate substrate levels by augmenting GLUT4 expression, while leaving the expression of GLUT1 and GLUT3 transporters unchanged. GLUT4, the insulin-sensitive glucose transporter, is largely found within muscle, adipose, and cardiac tissue. Physiologically, glucose-mediated insulin release results in increased GLUT4 expression and membrane translocation (Deng et al., 2015). Thus, Rhy prepares BM-hMSCs for prospective changes in cellular nutrient status through “metabolic flexibility.” HK is responsible for converting glucose to G6P, and PFK1 is the rate-limiting enzyme in the irreversible step of glycolysis (Almeida et al., 2004; Elabd et al., 2008). Rhy upregulates both mediators, amplifying the rate and maximum velocity of the glycolytic pathway (Almeida et al., 2004; Lunt and Vander Heiden, 2011). Importantly, Rhy does not affect levels of PK. The final metabolite of glycolytic metabolism, pyruvate, can be converted into acetyl-coenzyme A (CoA) by PDH or lactate by LDH. The reciprocal relationship that exists between these two enzymes will shunt pyruvate towards the TCA cycle and OXPHOS under conditions of enhanced mitochondrial enzyme activity (Vander Heiden et al., 2009; Kaplon et al., 2013). Alternatively, the reversible transformation of pyruvate into lactate is dependent on the ratio of nicotinamide adenine dinucleotide, NAD+ to NADH. Since the formation of lactate regenerates oxidized NAD+ and maintains glycolytic capacity by catalyzing the glyceraldehyde-3-phosphate (G3P)/1,3-biphosphoglyceric acid (1,3BPG) step of the pathway, an LDH blockade significantly impairs cellular proliferation (Vander Heiden et al., 2009). Conversely, shunting lactate out of the cell enhances cellular proliferation (Vander Heiden et al., 2009). Rhy is responsible for upregulating aerobic glycolysis by activating low density lipoproteins and increasing levels of ATP, while simultaneously inhibiting PDH; this Rhy-mediated amplification in glycolytic activity serves to support cellular proliferation of BM-hMSCs (Kaneko et al., 2020).
Rhy enhances the activity and protein expression of G6PD, the initial rate-limiting step of the pentose phosphate pathway, resulting in a higher proportion of phosphogluconolactonase to ribosose-5-phosphate (R5P) during the late G1 and S-phase of the cellular cycle (Jiang et al., 2011). During S-phase, aerobic glycolysis achieves maximal effect in mitogen-stimulated cells, while nascent protein production is severely downregulated (Lunt and Vander Heiden, 2011; Aviner et al., 2015). The PPP restores levels of reduced glutathione and thereby protects cellular proteins, lipids, and genomic nuclei acid against reactive oxygen damage (Kaneko et al., 2016). Reduced glutathione serves other key roles in lipid synthesis and formation of R5P, an essential metabolite in cellular proliferation. Acidic environmental conditions suppress the activity of G6PD, and thus, buildup of lactate inside the cell inhibits G6PD (Jiang et al., 2011). Rhy modulates this inhibition via lactate efflux through monocarboxylic transporters (lactate-proton symporter), which ultimately allows continual shunting of glucose towards the PPP, and subsequent amplification of protein expression (OPP), BrdU activity, and cellular proliferation (Kaneko et al., 2020).
Rhy, Warburg Effect, Stem Cells, and Conditioning Medicine
The ability to manipulate stem cell metabolism through Rhy treatment may allow for more efficient therapeutic strategies (Kaneko et al., 2020). Preconditioning with Rhy, or similar agents, may induce more favorable stem cell activity such as increased neurogenesis, proliferation, and differentiation, all of which warrant its investigation as a therapy. However, induction of Warburg Effect-like metabolism may be a more effective avenue to enhance stem cell activity in vivo. Astrocytes play an integral role in brain function through metabolic coupling with neurons, and their dysfunction plays a role in neurological diseases such as AD and epilepsy (Dossi et al., 2018). Research indicates that during metabolic stress, an astrocyte-to-neuron L-lactate shuttle (ANLS) system forms to support the energy-deprived neurons. ANLS is the process by which glycolytic astrocytes produce extracellular lactate to be taken up by neurons during the aglycemia apparent during neurodegeneration (Chamberlain and Sheng, 2019). Pre-conditioning stem cells to induce glycolytic metabolism and lactate production before transplantation may allow them to function as healthy astrocytes and aid in the maintenance of axonal integrity via ANLS and result in improved outcomes (Narayanan and Pérez-Pinzón, 2017).
The Warburg Effect may be the hallmark of cancer cells, however other cells exhibit similar metabolic activity. Endothelial cells (EC) play a crucial role in angiogenesis, a process that relies on the proliferation and transportation of cells into a perfused vessel (De Bock et al., 2013). Interestingly, ECs relied on aerobic glycolysis for 85% of their ATP, revealing that glycolysis was the focal bioenergetics pathway for proliferating ECs (Abdel-Haleem et al., 2017). The glycolytic flux of ECs was higher than other somatic cells and comparable to cancer cells. Valuable insight can be gained from ECs regarding their ability to proliferate and engraft into their target sight, and should be taken into account in future searches for a regenerative cell-based therapeutic option (Pradillo et al., 2019). An optimal glycolytic flux should be investigated to ensure increased proliferation and mitigate the chances of tumor formation. Notably, when BM-hMSC’s were treated with Rhy, there was no effect on the proto-oncogene, cMyc, accentuating its therapeutic potential (Kaneko et al., 2020), as tumorigenicity issues have become apparent in a few stem cell trials (Newman et al., 2005). Pre-conditioning stem cells with agents similar to Rhy and inducing aerobic glycolysis may give rise to favorable effects after transplantation. Recent literature has expanded upon various stem cell types as potentially advantageous therapeutics, including Muse cells, human amniotic fluid mesenchymal stromal cells, and human umbilical cord mesenchymal stem cells (Yu et al., 2009; Liu et al., 2013; Uchida et al., 2016). Further investigation on pre-conditioning agents and the Warburg Effect in non-cancerous cells is necessary to establish an optimal therapy to treat disease.
Rhy exhibits low solubility, low bioavailability, and low concentration in brain tissue (Xu et al., 2020); therefore coupling with stem cells may prove beneficial. Recently, Rhy-saturated nano-particles have been utilized as a vehicle to pass the blood brain barrier and attenuate effects of AD in mice, suggesting the efficacy of this coupling method (Xu et al., 2020). Rhy’s ability to mediate gene expression also makes it an appealing therapy to treat other diseases such as stroke. Rhy upregulates expression of BDNF in neuronal stem cells, which increases axon extension and synapse formation (Antonchick et al., 2013). This neurite outgrowth-promoting activity bolsters its potential as a stroke therapy. Use of Rhy-conditioned nano-particles and stem cells as a means of administration may enhance the ability to penetrate into areas of injury, however further examination of its interactions in other stem-cell combinations is imperative to optimize stem cell therapy (Acosta et al., 2014).
Stem cell aging is linked to metabolism and epigenetics (Ren et al., 2017). Altering stem cell metabolism may directly increase longevity, and indirectly manipulate epigenic activity, further reducing the chance of cellular exhaustion. The progression of many diseases ranging from cardiovascular disorders, neurodegenerative disorders, and cancer, can all be rooted back to cellular aging (Ren et al., 2017; Selvaraji et al., 2019). Treatment using pre-conditioned stem cells with manipulated metabolisms could help combat age-related disease. Stem cell mitochondria acquire mtDNA mutations and deletions over time, further exacerbating cellular exhaustion (Park and Larsson, 2011). Treatment with Rhy-like agents to induce the metabolic switch from OXPHOS to aerobic glycolysis may result in longer cellular lifespan and prolong aging and senescence, providing increased therapeutic efficacy.
Wrapping Up the Warburg Effects
Pre-conditioning stem cells with agents similar to Rhy allows for the unparalleled ability to manipulate cellular metabolism and activity. The findings reported above provide many fortuitous implications in investigating robust therapies for brain diseases. From stroke, to AD and epilepsy, the pretreatment of stem cells to alter cellular metabolism may furnish advantageous outcomes and mitigate the devastating effects of disease. The Warburg Effect is a fascinating mechanism with the potential to enhance cell survival and influence cellular activity, warranting further investigation in non-cancerous cells – stem cells. Further elucidation is crucial to optimize pre-treatment of stem cells with Rhy towards establishing safe and effective conditioning medicine regimens.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors thank the entire staff of the Borlongan Neural Transplant Laboratory for critical discussion of the manuscript.
References
Blaise Cozene1*
Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Bella Gonzales-Portillo1*
Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Jeffrey Farooq1
Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Cesar V Borlongan1
Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Yuji Kaneko1
Department of Neurosurgery and Brain Repair, University of South Florida Morsani College of Medicine, Tampa, Florida, USA.
Blaise Cozene and Bella Gonzales-Portillo contributed equally to this article.
Corresponding author:
Cesar V Borlongan
Email: cborlong@usf.edu

In a new window | Download PPT
Figure 1: Rhynchophylline affords its therapeutic effects through multiple mechanisms, including upregulation of BrdU, LDH, PFK1, G6PD, HK and cell proliferation, while downregulation biomass incorporation, nucleotide biosynthesis, and PDH production.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6366 | 12 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA