Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Whole-body heat shock accelerates recovery from impact-induced skeletal muscle damage in mice
Time:2020-09-17
Number:9689
Joshua S. Godwin1,2, Charles F. Hodgman1,2, Alan R. Needle1,3, Kevin A. Zwetsloot1,2, R. Andrew Shanely1,2
Author Affiliations


- 1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608.
- 2Integrated Muscle Physiology Laboratory, Boone; North Carolina, 28608.
- 3Injury Neuromechanics Laboratory, Boone; North Carolina, 28608.
Conditioning Medicine 2020. 3(4): 184-191.
Abstract
Impact-induced muscle damage (IIMD) is a common sports injury, often resulting in acute skeletal muscle contractile dysfunction. Whole-body heat shock (WBHS) is reported to attenuate skeletal muscle atrophy in animal models. The purpose of this study was to determine if WBHS prior to IIMD will attenuate subsequent muscle damage and accelerate recovery of contractile function. Skeletal muscle contractile function of the anterior crural muscle group was measured in adult male mice in vivo. Body temperature was then raised to 41°C (WBHS) or maintained at 37°C (normal body temperature, NBT) for 30 min. Twenty-four hours later a single impact was delivered to the tibialis anterior. Following 2-hours, 2-days, or 5-days of normal cage activity, contractile function was measured and the tibialis anterior was then excised and mounted for histological analysis. Two-hours post-IIMD contractile function was ~50% lower than pre-IIMD (p < 0.001, both groups), however, the WBHS group had greater maximal contractile function (p = 0.048). Two-days post-IIMD, the WBHS group had recovered (p = 0.090), but the NBT group had not recovered (p < 0.0001) contractile function. Five-days post-IIMD, WBHS group had fully recovered (p = 0.901), however the NBT group had not recovered (p < 0.0001) contractile function. Histological evaluation suggests less visible damage, and accelerated inflammatory response in the WBHS group. Immunohistochemistry results suggest IIMD increases HSP72 expression and this response is enhanced by prior WBHS. WBHS treatment prior to IIMD confers a degree of protection to skeletal muscle function 2-hours post-IIMD, attenuates muscle damage, and accelerates the rate of recovery of in vivo skeletal muscle contractile function within the 2-day and 5-day recovery period.
Keywords: Muscle injury, hyperthermia, inflammatory response, heat shock protein 72, skeletal muscle contractile function
Abstract
Impact-induced muscle damage (IIMD) is a common sports injury, often resulting in acute skeletal muscle contractile dysfunction. Whole-body heat shock (WBHS) is reported to attenuate skeletal muscle atrophy in animal models. The purpose of this study was to determine if WBHS prior to IIMD will attenuate subsequent muscle damage and accelerate recovery of contractile function. Skeletal muscle contractile function of the anterior crural muscle group was measured in adult male mice in vivo. Body temperature was then raised to 41°C (WBHS) or maintained at 37°C (normal body temperature, NBT) for 30 min. Twenty-four hours later a single impact was delivered to the tibialis anterior. Following 2-hours, 2-days, or 5-days of normal cage activity, contractile function was measured and the tibialis anterior was then excised and mounted for histological analysis. Two-hours post-IIMD contractile function was ~50% lower than pre-IIMD (p < 0.001, both groups), however, the WBHS group had greater maximal contractile function (p = 0.048). Two-days post-IIMD, the WBHS group had recovered (p = 0.090), but the NBT group had not recovered (p < 0.0001) contractile function. Five-days post-IIMD, WBHS group had fully recovered (p = 0.901), however the NBT group had not recovered (p < 0.0001) contractile function. Histological evaluation suggests less visible damage, and accelerated inflammatory response in the WBHS group. Immunohistochemistry results suggest IIMD increases HSP72 expression and this response is enhanced by prior WBHS. WBHS treatment prior to IIMD confers a degree of protection to skeletal muscle function 2-hours post-IIMD, attenuates muscle damage, and accelerates the rate of recovery of in vivo skeletal muscle contractile function within the 2-day and 5-day recovery period.
Keywords: Muscle injury, hyperthermia, inflammatory response, heat shock protein 72, skeletal muscle contractile function
Introduction
Impact-induced skeletal muscle damage (IIMD), or contusion, is typically caused by high-energy blunt force, non-penetrating trauma; and depending on the severity of the injury, may take days to weeks to resolve (Diaz et al., 2003; Naughton et al., 2018). IIMD may result in edema, overt structural damage, contractile dysfunction, and is resolved by repair. The current standard of care for IIMD is the RICE technique (rest, ice, compression, and elevation). Acutely, RICE attenuates pain, slows tissue metabolism, limits the inflammatory response, decreases markers of oxidative stress, and promotes vasoconstriction (Swenson et al., 1996; Puntel et al., 2011; Naughton et al., 2018). However, icing after IIMD has been reported to delay regeneration (Singh et al., 2017), thus calling the long-term efficacy of this technique into question. Other IIMD treatment strategies have been explored; however, these have not advanced the current standard of care (Naughton et al., 2018).
The heat shock family of stress proteins (HSPs) are upregulated when an organism is exposed to whole body “stress” stimuli, such as heat or oxidative stress, whole body exercise, and in vivo eccentric contractions (Lindquist, 1986; Locke et al., 1990; Ingalls et al., 1998). In mammals, the cytoprotective roles of HSP induction have been historically studied in the context of myocardial ischemia-reperfusion (Currie et al., 1988; Vass et al., 1988). Within two hours of whole-body heat shock (WBHS) the inducible form of the 70-kDa family of HSPs (HSP72) is upregulated in skeletal muscle (McArdle and Jackson, 1996). Upregulation of HSP72 in skeletal muscle confers protection from multiple forms of skeletal muscle injury. WBHS prior to skeletal muscle disuse atrophy attenuates muscle loss and oxidative stress (Naito et al., 2000; Selsby and Dodd, 2005; Yoshihara et al., 2015). Additionally, induction of HSP72 prior to skeletal muscle injury attenuates loss of contractile function after eccentric contractions, reloading injury following disuse, and disuse contractile dysfunction (McArdle et al., 2004; Senf et al., 2013; Yoshihara et al., 2015). Chemically induced skeletal muscle injury results in rapid loss of structure and function followed by regeneration. A previous report indicates the absence of HSP72 expression in chemically injured skeletal muscle results in sustained inflammation and abnormal repair, whereas forced expression of HSP72 restores the normal inflammatory and repair response (Senf et al., 2013). Collectively, the literature suggests that activation of HSPs can attenuate many types of muscle damage and promote repair. Therefore, the purpose of this study was to test the following hypothesis: WBHS prior to IIMD will attenuate IIMD and accelerate recovery of contractile function.
Methods and materials
Experimental Design. Male C57BL/6 mice aged 12-to-14 months, procured from the in-house colony at Appalachian State University, were randomly assigned to one of six groups: WBHS 2-hour (n = 8), WBHS 2-day (n = 9), WBHS 5-day (n = 9), normal body temperature (NBT) 2-hour (n = 9), NBT 2-day (n = 8), and NBT 5-day (n = 9). Mice were anesthetized (4% isoflurane and maintained with 2% isoflurane) and pre-IIMD in vivo contractile function of the anterior crural muscle group (tibialis anterior, extensor digitorum longus, and extensor hallucis longus) was measured. Mice were then subjected to WBHS or maintained at NBT, allowed to recover consciousness from anesthesia, and then returned to normal cage activity. Twenty-four-hours later, mice were re-anesthetized and received a single impact to the left tibialis anterior muscle using the mass-drop method for IIMD. All mice were then allowed to recover consciousness and were returned to normal cage activity. Following the assigned recovery time (2-hours, 2-days, or 5-days), mice were re-anesthetized and post-IIMD in vivo contractile function was measured. After measuring post-IIMD contractile function (repeated measures design), the tibialis anterior muscle was harvested and mounted for histological analysis. This study was approved by the Appalachian State University IACUC (protocol #16-18). All procedures were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
In Vivo Contractile Function. Skeletal muscle contractile function was measured pre- and post-IIMD in the same mouse via the torque-frequency relationship, as previously described (Lowe et al., 1995; Corona et al., 2008). Under anesthesia (4% isoflurane inhalation and maintained with 2% isoflurane inhalation), hair on the left hind leg was removed with dilapidation cream, rinsed with water, and disinfected with povidone-iodine. Mice were then placed on a heated (37°C) platform. The left foot was secured with an aluminum foot-cover and flexible medical wrapping to the footplate affixed to the shaft of a dual-mode servomotor (300B-LR, Aurora Scientific, Aurora, ON, Canada). A clamp secured to a micro-manipulator (World Precision Instruments, Sarasota, FL) was used to position and hold the left knee in place during the procedure. The ankle joint was held at 90° of passive dorsiflexion with respect to the tibia and the tibia was positioned at 90° with respect to the femur. Sterilized 30-gauge needle electrodes (Grass Instruments, Warwick, RI) were inserted through the skin for stimulation of the left common peroneal nerve and each was positioned and held in place with a micro-manipulator (World Precision Instruments, Sarasota, FL). Isometric twitch (1 Hz) contractions were used to obtain initial needle electrode placement, and optimal stimulation voltage (5-10 volts) and needle electrode placement were confirmed by 5-10 isometric contractions (200-ms train duration, 0.1-ms pulse width at 300 Hz). Following needle electrode placement, a torque-frequency curve measured peak isometric torque produced by the anterior crural muscle group at 10 ascending stimulation frequencies, 20 Hz to 300 Hz, with two minutes rest between each contraction. The peak twitch-to-tetanic ratio (Pt/Po), peak torque generated during a twitch (20 Hz) expressed as fraction of maximal tetanic torque (250 Hz), was also measured.
Whole-body Heat Shock. Under anesthesia (via isoflurane inhalation), all mice were placed on an electric heating pad in the prone position. Body temperature of the WBHS groups were raised to 41oC for 30 minutes and continuously monitored by a rectal temperature probe (Harvard Apparatus, Holliston, MA). Mice in the NBT groups were maintained at 37oC for 30 minutes and continuously monitored by a rectal temperature probe. All mice were then allowed to fully recover consciousness from anesthesia and resume normal cage activity. Preliminary data indicates that WBHS does not affect in vivo contractile function, data not shown.
Injury Model. The mass-drop technique described by Crisco et al. (1994) and modified for mice by Xiao et al. (2016) was used to induce IIMD. Specifically, 24-hours after WBHS or NBT treatment, mice were anesthetized (via isoflurane inhalation) and a 14.1g steel ball was dropped from a height of 115 cm through a PVC tube onto a metal impactor that directly impacts the belly of the tibialis anterior (TA) without perforating the skin or breaking the tibia or fibula.
Data Acquisition and Analysis. The muscle lever system (Aurora Scientific 1300A, Aurora, ON, Canada), stimulator, and force transducer were connected to a signal interface (Aurora Scientific, Model 610A) that sends the analog signal to an analog-to-digital converter card (National Instruments, Austin, TX) on a computer with Dynamic Muscle Control software (Aurora Scientific, 610A). The force output data were analyzed utilizing Dynamic Muscle Analysis software (Aurora Scientific, 610A).
Histology. The TA was harvested and mounted for histological analysis following measurement of post-IIMD in vivo contractile function. Harvested tissue was mounted on cork using optimal cutting temperature medium (Fisher Scientific, Houston, TX), frozen in isopentane-cooled in liquid nitrogen. Slide-mounted cross-sections, 10 μm thick, were obtained. Mounted tissue sections were then stained using common histological techniques for cytosolic and nucleic components using Mayer’s hematoxylin and eosin (H&E) solution (Millipore Sigma, St. Louis, MO). After drying fully, coverslips were applied to the slides using Vectashield Hardset (Vector, H-1500). Approximately 300 fibers/section were imaged with 10x - 20x objectives using an Olympus IX81 light microscope and cellSens Imaging Software (Olympus, Waltham, MA). Qualitative and quantitative indices of muscle damage included edema and overt fiber damage and the presence of one or more of the following: extracellularly-located leukocytes, pale cytoplasm, intracellularly-located leukocytes, and centrally-located myonuclei (Koh and Brooks, 2001; Tsivitse et al., 2003). The total number of damaged/regenerating fibers was reported as a percentage of the total number of fibers within each cross section.
Fluorescent identification of HSP72 in TA muscle sections (3-4 samples per group in the 2-hour timepoint) was performed as follows. Sample slides were removed from the -80°C freezer, allowed to warm up to room temperature, and tissue sections were encircled using a hydrophobic-barrier PAP pen (Vector, Burlingame, CA; ImmEdge pen). Samples were blocked by incubating with 10% normal goat serum (NGS; Vector, S-1000) in wash buffer (1X phosphate buffered saline containing 0.04% triton X-100) for one hour at room temperature. Samples were then incubated overnight with HSP70/HSP72 primary antibody (Enzo Life Sciences; Farmingdale, NY; Cat # ADI-SPA-812-D) at 1:1000 in 10% NGS overnight at 4°C in the dark. Following primary antibody incubation, samples were washed for 5x5 minutes with wash buffer. Next, samples were incubated with a goat anti-rabbit secondary antibody conjugated to FITC (Novus, A16118) at 1:2500 in 10% NGS solution for one hour at room temperature in the dark. Samples were washed again for 5x5 minutes using wash buffer. After drying fully, coverslips were applied to the slides using Vectashield Hardset (Vector, H-1500). Samples were imaged using the EVOS® FL Imaging Station (Life Technologies, Carlsbad, CA).
Statistics. All data are expressed as means ± SEM. The data were analyzed using a repeated measures three-way factorial ANOVA (condition, two levels; time, three levels; stimulation frequency, 10 levels). The percent of injured fibers data were analyzed using a one-way ANOVA. The a priori level of significance was set at p < 0.05 and Fisher’s LSD pair-wise comparisons were made post hoc. Data were analyzed using SPSS (IBM Corp., Armonk, NY).
Results
Contractile Function. Contractile function was measured 2-hours post-IIMD to assess the early/acute injury phase. At 2-hours post-IIMD, there was a significant time-by-frequency-by-group interaction (F = 6.001, p = 0.0001). The torque-frequency relationship at 2-hours was significantly depressed in both treatment groups (F = 4.075, p = 0.001 for both groups); however, the contractile function in the WBHS-treated mice was significantly greater than NBT mice (F = 4.677, p = 0.048, Figure 1 A). Analysis of torque at a submaximal (40 Hz) and a maximal tetanic frequency (250 Hz) revealed that torque at both frequencies was significantly depressed, compared to pre-injury for both groups, (WBHS, F = 79.628, p = 0.0001; NBT, F = 104.462, p = 0.0001). However, the WBHS group generated significantly more torque than the NBT group at 250 Hz, but not at 40 Hz (F = 4.677, p = 0.048; F = 1.249, p = 0.195, respectively, Figure 2 A, B). A significant time-by-group interaction occurred in Pt/P0 (F = 6.642, p = 0.0001). At 2-hours post-IIMD the Pt/P0 was significantly depressed in the NBT and WBHS treatment groups (F = 31.782, p = 0.001; F = 23.267, p = 0.001, respectively) but did not differ between groups, p = 0.054 (Figure 2 C).
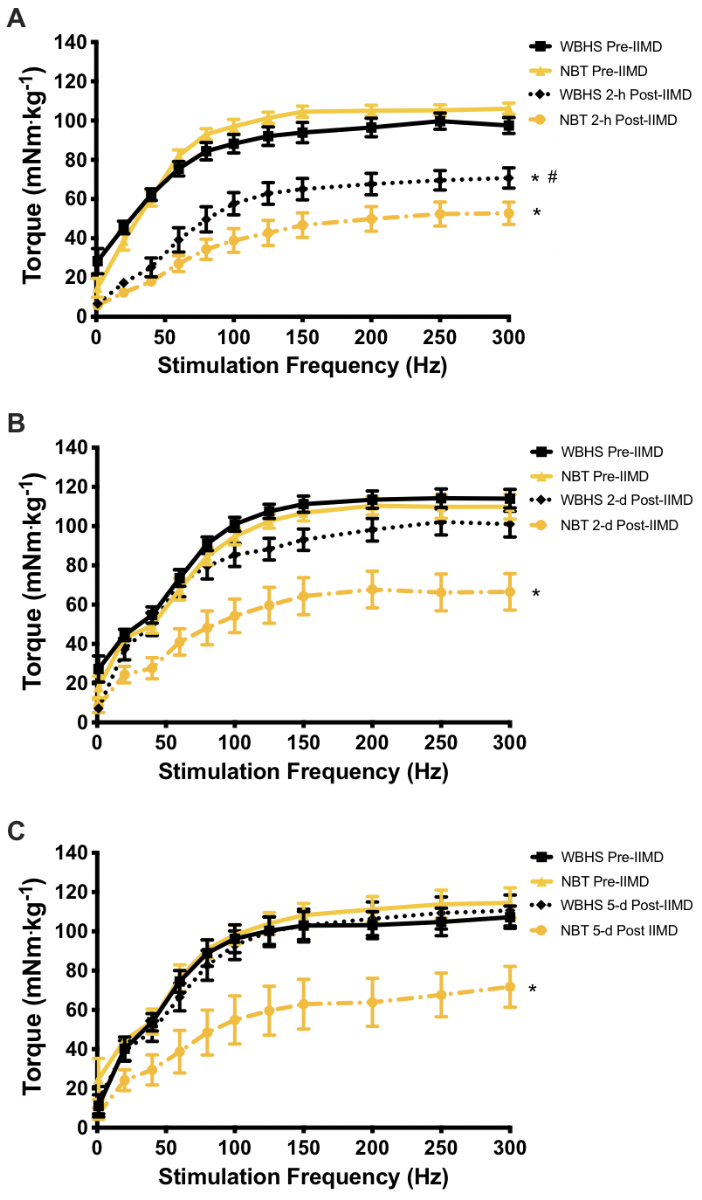
In a new window | Download PPT
Figure 1: In vivo torque-frequency relationship pre-impact induced muscle damage (IIMD) and post-IIMD. Whole-body heat shock (WBHS); normal body temperature (NBT). A) Pre-impact and 2-hours post-IIMD. B) Pre-impact and 2-days post-IIMD. C) Pre-impact and 5-days post-IIMD. *p < 0.05 within group, pre-IIMD vs post-IIMD. , # p < 0.05 between treatment groups, within time point. Data are means ± SEM.
Given these revealing findings, we next assessed muscle contractile function at 2-days post-IIMD. A significant time-by-frequency-by-group interaction effect was also measured at this time point (F = 3.689, p = 0.024). WBHS-treated mice recovered torque values over the entire torque-frequency relationship (F = 3.308, p = 0.090), whereas NBT mice did not (F = 23.071, p = 0.0001, Figure 1 B). Analysis of torque at 40 Hz and 250 Hz revealed that WBHS-treated mice recovered torque at both frequencies by 2-days (F = 0.444, p = 0.516; F = 1.675, p = 0.217, respectively), while the NBT mice did not (F = 9.244, p = 0.009; F = 21.583, p = 0.0001, respectively, Figure 2 A, B). The Pt/P0 recovered to pre-IIMD levels in the NBT and WBHS treatment groups (F = 0.713, p = 0.403; F = 0.040, p = 0.843, respectively, Figure 2 C) as a result of proportional recovery of torque at 20 Hz and 250 Hz.
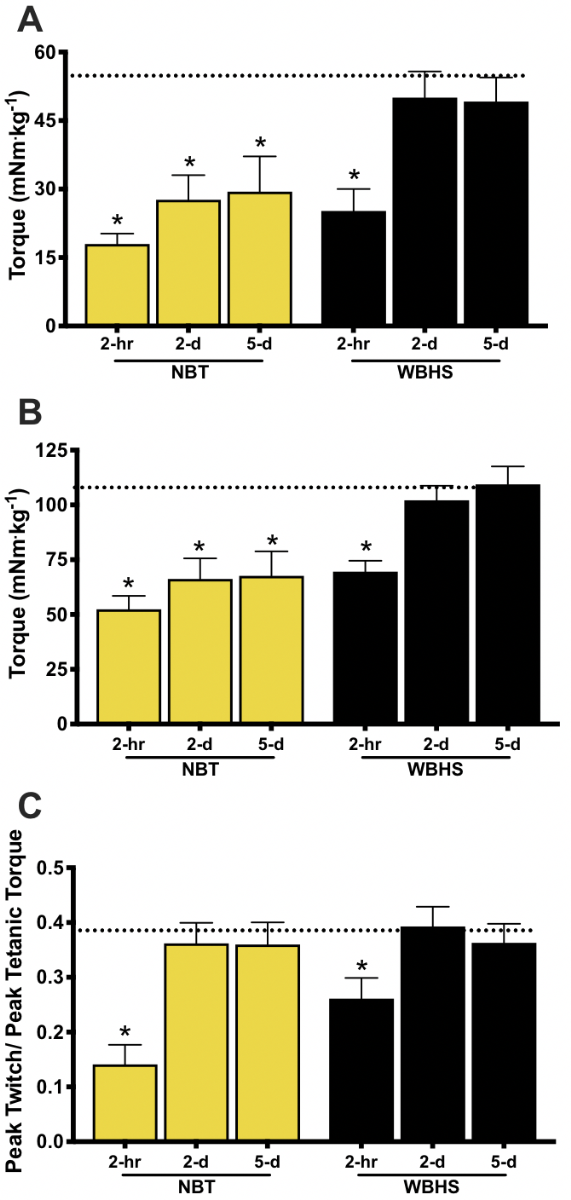
In a new window | Download PPT
Figure 2: Submaximal and maximal in vivo torque and peak twitch/peak tetanic torque ratios. A) Submaximal 40 Hz stimulation frequency for both treatment groups at all time points. B) Maximal 250 Hz stimulation frequency for both treatment groups at all time points. C) Peak twitch (20 Hz) to maximal tetanic torque ratio (Pt/Po). The dotted line represents the average pre-impact induced muscle damage (IIMD) value for all treatment groups; normal body temperature (NBT), whole-body heat shock (WBHS). * p < 0.05 pre-IIMD vs post-IIMD, within group. Data are means ± SEM.
According to the data reported by Crisco et al., (1994) contractile function at 7-days post IIMD is recovering, but remains depressed. Consistent with Crisco et al., (1994) we report that a significant time-by-frequency-by-group interaction effect occurred at 5-days post-IIMD (F = 3.602, p = 0.038). Mice that received WBHS treatment fully recovered torque values over the entire torque-frequency relationship (F = 0.016, p = 0.901), whereas mice that were maintained at NBT did not (F = 26.368; p = 0.0001, Figure 1 C). As at 2-days post-IIMD, torque at 40 Hz and 250 Hz in the WBHS-treated mice had recovered (F = 0.078, p = 0.786; F = 0.293, p = 0.602; respectively), whereas torque in the NBT group had not recovered (F = 23.943, p = 0.001; F = 12.759, p = 0.006, respectively, Figure 2 A, B). The proportional recovery of torque at 20 Hz and 250 Hz remained in the NBT and WBHS treatment groups, thus Pt/P0 did not differ from baseline (F = 0.465, p = 0.499; F = 0.098, p = 0.756, respectively, Figure 2 C).
Histology. H&E staining was used to assess changes in skeletal muscle morphology following IIMD. At 2-hours post-IIMD, both WBHS and NBT groups had apparent edema and fiber disruption; however, the edema in NBT groups appeared to be more pronounced than WBHS (Figure 3 B and C). Presence of extracellular-located leukocytes were observed in both NBT and WBHS groups at 2-hours post-IIMD. At 2-days post-IIMD, extracellular leukocytes were present in both groups, with a more pronounced presence of intracellular leukocytes present in the NBT group (Figure 3 D and E). Edema and fiber disruption were still present at 2-days in the NBT group, while WBHS appeared to have less edema. Centrally-located myonuclei were present in the WBHS group 2-days post-IIMD, while few were observed in the NBT group. Conversely, the presence of pale cytoplasm was present in both groups at this time point post-injury. At 5-days post-IIMD, the NBT group displayed edema and fiber disruption, with minimal presence of these injury markers in the WBHS group (Figure 3 F and G). Centrally-located myonuclei were highly present in the NBT group and few central myonuclei were observed in the WBHS group at 5-days post-IIMD. Further, the presence of extracellular located leukocytes was elevated in the NBT group at 5-days post-IIMD, while very few were observed in the WBHS group. The percentage of injured myofibers fibers displayed a significant main effect of group (F = 7.395; p = 0.0001). Post hoc analysis revealed the percent of injured fibers did not differ between groups at 2-hours, or 2-days post-IIMD (p = 0.501, and p = 0.342, respectively). However, the NBT group at 5-days post-IIMD had significantly more injured fibers than the WBHS group 5-days post-IIMD, (p = 0.048, Figure 4).
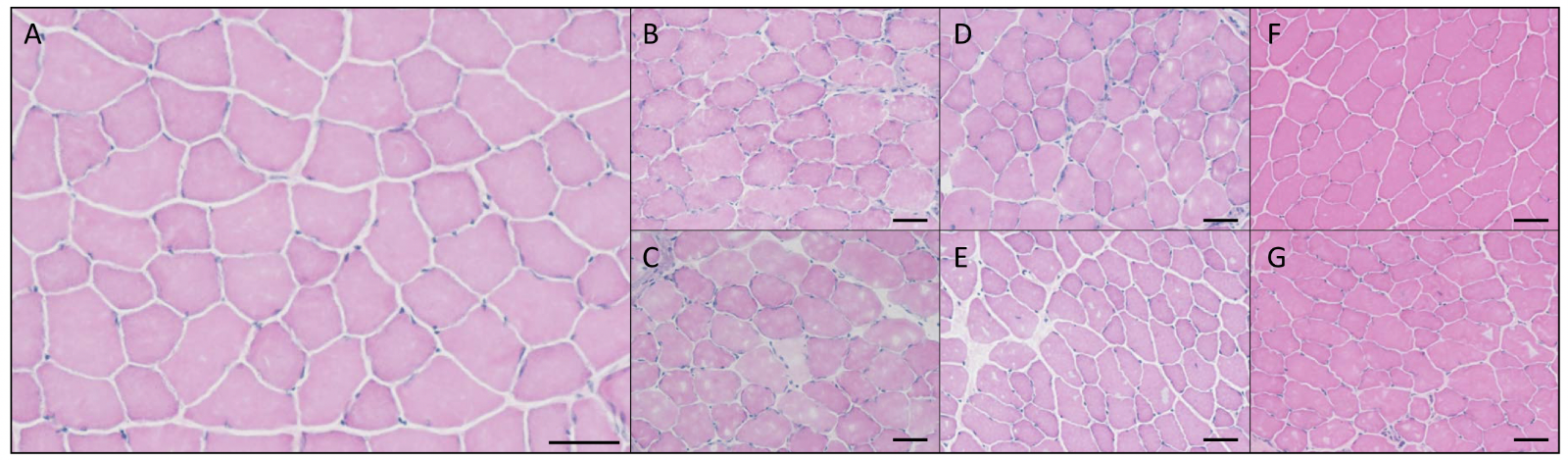
In a new window | Download PPT
Figure 3: Representative hematoxylin and eosin (H&E) stain, tibialis anterior myofibers, 20X magnification, scale bar represent 50 μm. A) uninjured, control. B) whole body heat shock (WBHS) 2-hour post-IIMD. C) Normal body temperature (NBT) 2-hours post-IIMD. D) WBHS 2-days post-IIMD. E) NBT 2-days post-IIMD. F) WBHS 5-days post-IIMD. G) NBT 5-days post-IIMD.

In a new window | Download PPT
Figure 4: Total number of damaged/regenerating fibers calculated as a percentage of the total number of fibers that demonstrated overt signs of injury. Control (Con), normal body temperature (NBT), whole body heat shock (WBHS). * p < 0.05 from control uninjured tibialis anterior muscle; # p < 0.05 between treatment groups, within time point; ‡ p < 0.05 within treatment group. Data are means ± SEM.
HSP72 expression was visualized by immunohistochemistry. Basal expression of HSP72 is very low in the uninjured, NBT, TA myofibers (Figure 5 A). In uninjured TA myofibers, HSP72 expression was increased 26-hours after exposure to WBHS (Figure 5 B). IIMD increased the expression of HSP72 in TA myofibers (Figure 5 C) and was similar to that of uninjured WBHS myofibers. WBHS 24-hours prior to IIMD augmented HSP72 expression in the myofibers (Figure 5D).
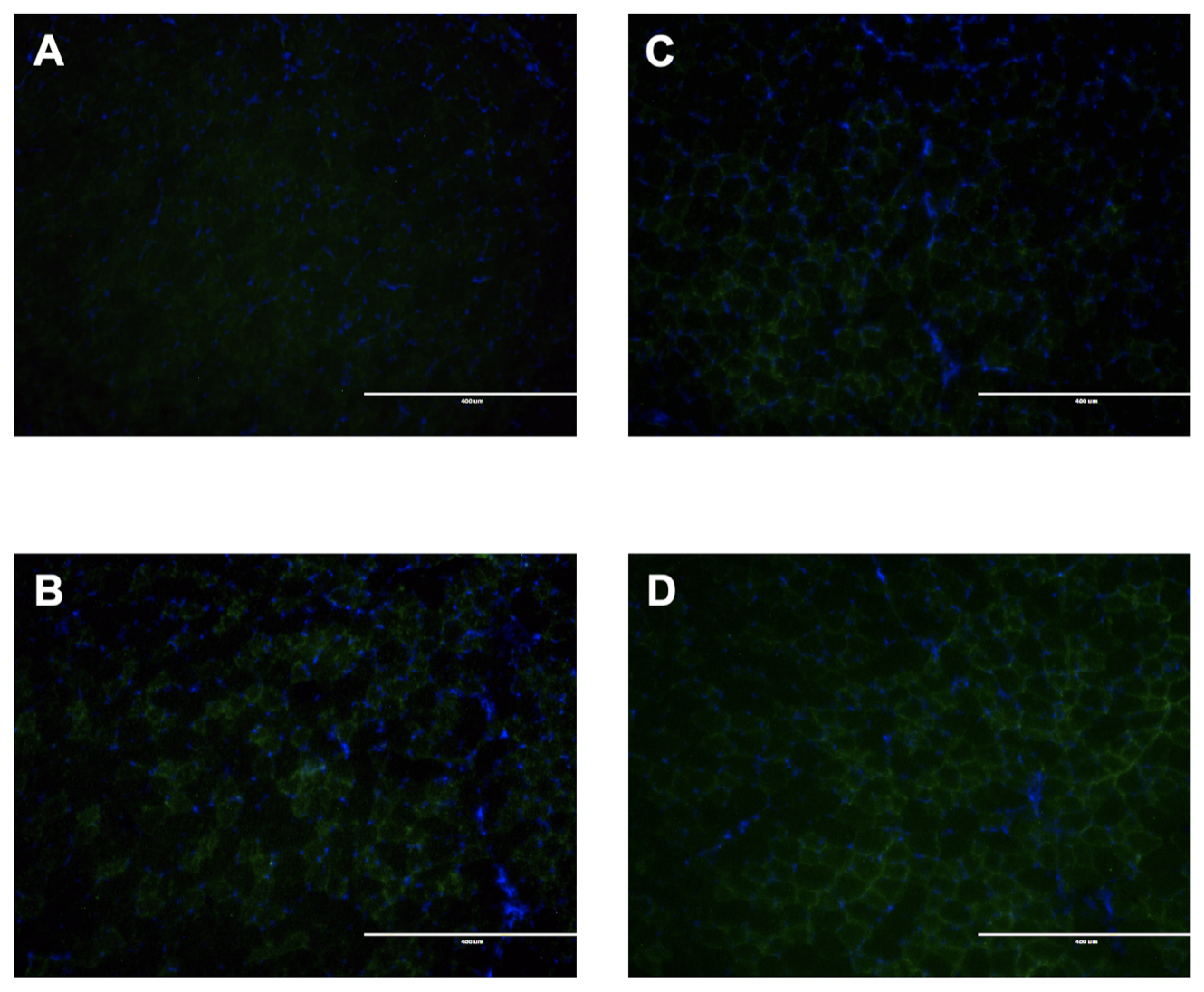
In a new window | Download PPT
Figure 5: Representative HSP72 expression (green) and nuclei (blue) in tibialis anterior myofibers, 10X magnification, scale bar represent 400 μm. Immunohistochemical staining was performed on A) normal body temperature (NBT), uninjured; B) 26-hours post-WBHS, uninjured; C) NBT, 2-hours post-IIMD; and D) 26-hours post-WBHS, 2-hours post-IIMD tibialis anterior myofibers.
Discussion
This is the first study to test the hypothesis that WBHS 24-hours prior to IIMD will attenuate subsequent muscle damage and accelerate recovery of contractile function. The early findings of this study support our hypothesis. Specifically, contractile function returned to baseline within 2-days of injury, fewer intracellularly-located leukocytes were observed at 2-days, and fewer centrally-located myonuclei were observed 5-days after injury in the WBHS-treated mice. Taken together, these findings suggest that preconditioning mice with WBHS is effective at accelerating the recovery of contractile function and attenuating muscle damage in response to IIMD.
Accelerated Recovery of In-Vivo Contractile Function. To our knowledge, this is the first study to measure contractile function pre- and post-IIMD in the same animals. Our pre-IIMD (baseline) in vivo anterior crural muscle contractile function data are supported by previous reports (Lowe et al., 1995; Corona et al., 2008). IIMD resulted in depressed contractile function across the torque frequency relationship in the NBT group, with deficits in maximal torque of ~50% at 2-hours and ~40% at 2- and 5-days post-IIMD (Figure 1). These post-IIMD contractile function data are supported by previous IIMD studies (Crisco et al., 1994; Delos et al., 2014; Russ et al., 2018).
WBHS treatment, 24-hours prior to IIMD, accelerated recovery of contractile function across the torque-frequency relationship. Two-hours post-IIMD, the torque-frequency relationship was significantly depressed in both treatment groups. However, WBHS treatment attenuated the loss of function at higher stimulation frequencies (Figure 1). At the submaximal stimulation frequency of 40 Hz, function did not differ between treatment groups 2-hours post-IIMD (Figure 2 A). However, at 250 Hz, WBHS treatment attenuated the loss (-30%) in torque compared to NBT mice (-50%; Figure 2 B). These novel data suggest WBHS dampened IIMD. Over expression of HSP70 in the skeletal muscles of transgenic mice has been reported to accelerate recovery of contractile function following eccentric (lengthening contractions) muscle damage over a 28-day recovery period (McArdle et al., 2004). Our acute post-injury data differ from McArdle et al. (2004) wherein WBHS attenuated the decrease in contractile function measured 2-hours after injury and lifelong overexpression of HSP70 did not attenuate the loss in contractile function 3-hours after eccentric damage. Our data suggest, the WBHS group recovered contractile function within 2-days of IIMD (Figure 2 B). Similarly, McArdle et al. (2004) report lifelong overexpression of HSP70 enhanced recovery 3-days and 14-days post-eccentric damage and full recovery 28-days post-injury. Previous skeletal muscle atrophy-induced contractile dysfunction studies (Ichinoseki-Sekine et al., 2014) report a single bout of WBHS attenuates atrophy, but not the loss of contractile function.
Contractile dysfunction resulting from IIMD may be due in part to impaired excitation-contraction (EC) coupling and/ or degradation of structural and contractile proteins. Russ et al. (2018) reported oxidative stress and a loss of calcium homeostasis following IIMD. Contractile dysfunction occurs during periods of oxidative stress and is related to oxidation of the myofilaments, ryanodine receptor, and the sarcoplasmic/endoplasmic Ca2+-ATPase (SERCA) (Scherer and Deamer, 1986; Anzai et al., 2000; Smith and Reid, 2006). The Pt/P0 is an indirect measure of EC-coupling reflecting changes in Ca2+ sensitivity and/or Ca2+ release from the sarcoplasmic reticulum. IIMD resulted in a significant decrease in Pt/P0 in both treatment groups 2-hours post-injury that returned to baseline by 2-days post-injury. The initial decrease in Pt/P0 suggests IIMD may cause an impairment in EC-coupling. However, recovery of Pt/P0 to baseline in both groups 2-days post-injury while indices of damage remained elevated in the NBT group suggests another mechanism is responsible for the decrement in contractile function. HSP72 is reported to maintain ryanodine receptor and SERCA function during periods of stress (Tupling et al., 2004; Liu et al., 2006). Gehrig et al. (2012) reported that upregulation of HSP72 partially restores both SERCA and contractile function in dystrophic mice, a model of muscle degeneration. Further, the loss of Ca2+ homeostasis results in increased cytosolic Ca2+ levels and subsequent activation of calpains and Ca2+ activated proteases, which degrade cytoskeletal and contractile proteins (Goll et al., 2003), resulting in contractile dysfunction. Induction of HSP72, via WBHS, may attenuate Ca2+ dyshomeostasis resulting from IIMD, thus reducing the loss of cytoskeletal and contractile proteins. However, the mechanisms by which WBHS attenuates the immediate loss of skeletal muscle contractility and accelerates recovery from a single injury (IIMD) remain to be elucidated.
Histological Analysis. Following muscle injury, invasion of phagocytic and non-phagocytic immune cells is required for regeneration and recovery of normal structure and function. In the current study, quantitative morphological markers of muscle damage included the presence of pale cytoplasm, extracellular leukocytes, intracellular leukocytes, and centrally-located myonuclei, as previously described (Koh and Brooks, 2001; Tsivitse et al., 2003). In general, our histological observations are supported by previous IIMD studies of overt muscle fiber damage, edema and subsequent infiltration of leukocytes (Crisco et al., 1994; Kruger and Smith, 2012; Myburgh et al., 2012; Xiao et al., 2016) and essentially aligns with the typical time course that follows after skeletal muscle trauma (Tidball, 2017).
Two-hours post-IIMD both WBHS and NBT groups had pronounced fiber disruption and edema. The presence of fiber disruption and edema at the 2-hour time point is consistent with previous studies (Crisco et al., 1994; Kruger and Smith, 2012; Myburgh et al., 2012). The percentage of damaged fibers did not differ between treatment groups (Figure 4). Extracellular leukocytes were equally present in both groups at this time point. The extracellular leukocytes are expected to be neutrophils, the first immune cells to invade damaged tissue following an injury (Tidball, 2017). Our NBT observations at this time point are comparable to previous studies (Kruger and Smith, 2012; Myburgh et al., 2012; Xiao et al., 2016). However, in this study, WBHS prior to IIMD did not affect the appearance of extracellular leukocytes to the site of the injury. Previous reports indicate WBHS increases the expression of HSP72 in skeletal muscle, in vascular tissue, and in exosomes released from peripheral blood mononuclear cells (Lancaster and Febbraio, 2005; Kami et al., 2019). HSP72 has chemoattractant effects on neutrophils, stimulating neutrophil chemotaxis (Ortega et al., 2009). Thus, WBHS prior to IIMD was expected to result in a greater leukocyte response at this time point. We do not have an explanation for these divergent results. Further studies are required to elucidate this issue.
Fiber disruption and edema are evident in both groups 2-days post-IIMD; however, the degree of fiber disruption and edema appeared to be more pronounced in the NBT group. It has been proposed that fiber damage and edema disrupt actin and myosin filament interaction, thereby decreasing the ability of cross-bridges to form, resulting in decreased contractile function (Crisco et al., 1994; Naughton et al., 2018). Thus, IIMD-induced edema may be a contributing factor to the greater deficit in contractile function observed in the NBT group. The percentage of damaged fibers was significantly elevated in the NBT group, compared to control and WBHS, 2-days post-IIMD. Pale cytoplasm, a marker of degenerating myofibers (Sewry and Goebel, 2013), has not been reported in previous IIMD studies. The greater number of degenerating myofibers observed in the NBT group may also be a contributing factor to the post-IIMD contractile dysfunction. Extracellular and intracellular leukocytes were present in both groups at this time point, though more leukocytes appeared present in the NBT group. Pro-inflammatory (cytolytic, phagocytic) M1-like macrophages remove debris and regulate muscle repair and regeneration (Tidball, 2017). Previous studies report elevated M1-like macrophages in the 1-3 days post-IIMD timeframe (Myburgh et al., 2012; Xiao et al., 2016). The transition from predominantly M1-like macrophages to anti-inflammatory (non-phagocytic) M2-like macrophages is well orchestrated, wherein M2-like macrophages deactivate M1-like macrophages and stimulate the later stages of muscle repair and regeneration (Tidball, 2017). Thus, a proportion of intracellular leukocytes observed in this study are expected to be M2-like macrophages (Xiao et al., 2016). Centrally-located myonuclei are a primary marker for regenerating muscle fibers following an injury (Tidball, 2017). In the WBHS group, centrally located myonuclei were observed, while few were present in the NBT group 2-days post-IIMD.
Five-days post-IIMD, fiber disruption and edema remained apparent in the NBT group (though less than at day-2), while this was nearly resolved in the WBHS group. The continued presence of fiber disruption and edema in the NBT group is in agreement with previous IIMD studies (Kruger and Smith, 2012; Myburgh et al., 2012). The percentage of damaged fibers remained significantly higher in the NBT group, compared to control and WBHS, 2-days post-IIMD. Extracellularly-located leukocytes continued to be elevated in the NBT group, whereas few were observed in the WBHS group. The presence of extracellularly located leukocytes in this study differs from Xiao et al. (2016) who reported baseline neutrophil levels 5-days post-IIMD. Five-days post-IIMD, we observed very few intracellularly located leukocytes in both groups. Our observation differs from a previous IIMD study indicating M2-like macrophages remain elevated 5-days post-IIMD (Xiao et al., 2016). It is not clear why our leukocyte data differ from previous IIMD studies. Centrally located myonuclei were abundant in the NBT group, while few were present in the WBHS group. The elevated presence of centrally located myonuclei in the NBT group in this time frame is in line with previous studies (Myburgh et al., 2012; Xiao et al., 2016). Previous studies demonstrate HSP72 expression is required for skeletal muscle to mount a normal inflammatory and regeneration response following injury (Senf et al., 2013). Further, an accelerated inflammatory and repair response has been reported when heat stress is applied to skeletal muscle after crush- or cardiotoxin-induced injury (Takeuchi et al., 2014; Shibaguchi et al., 2016; Kami et al., 2019). We postulate that the accelerated recovery of contractile function that we observed in the WBHS group may be due to less damage incurred and an accelerated inflammatory and repair response. Our findings suggest there was less initial damage in the WBHS group, evident by the elevated contractile function data 2-hours post-IIMD (Figure 3 A). Less initial damage would result in a decreased leukocyte response and fewer regenerating fibers, resulting in fewer centralized myonuclei. The crush- or cardiotoxin-induced injury reports (Takeuchi et al., 2014; Shibaguchi et al., 2016; Kami et al., 2019) suggest the upregulation of HSP72 by WBHS may accelerate both the inflammatory response and regeneration in response to IIMD.
The stress-inducible isoform of HSP70 (HSP72) has little to no expression in unstressed cells, it is however, highly inducible upon exposure to various forms of stress. Low expression levels of HSP72 were found in the NBT, uninjured TA (Figure 5 A) and this is supported by previous reports. HSP72 expression is predominately expressed in type I and IIa fiber types in sedentary, uninjured muscle (Neufer et al., 1996; Oishi et al., 1998) and the TA has very few of these fibers (Hamalainen and Pette, 1993; Kammoun et al., 2014). We observed IIMD, in the absence of WBHS, increased HSP72 expression in the injured TA harvested 2-hours post-IIMD (Figure 5 C) and to our knowledge, this is the first such report. Our novel finding is supported by a previous report by Ingalls et al. (1998). They found HSP72 induction within 6-hours of in vivo eccentric muscle contractions in the TA (rectal temperature is maintained at 37oC) and HSP72 expression remained elevated 14-days post-injury (Ingalls et al., 1998). WBHS is widely reported to increase HSP72 expression in skeletal muscle. Our 26-hour post-WBHS levels (Figure 5 B) are in line with the early report published by McArdle and Jackson (1996). They report that WBHS increases expression of HSP72 in skeletal muscle within 2-hours, peaks at approximately 18 hours, and returns to close to baseline levels 48-hours post-WBHS (McArdle and Jackson, 1996). We found that WBHS 24-hours prior to IIMD augments HSP72 expression in the injured TA (Figure 5 D). Organisms upregulate HSPs when exposed to various “stress” stimuli (Lindquist, 1986; Locke et al., 1990; Ingalls et al., 1998). The family of HSP70s are ATP-dependent molecular chaperones with wide-ranging physiological functions including chaperoning nascent proteins as they exit the ribosome to prevent misfolding, refold stress-unfolded proteins, and assist in post-translational translocation of proteins across membranes or the proteolytic machinery (Clerico et al., 2015). A population of muscle fibers that were reversibly damaged by IIMD may be repaired more readily due to the increased HSP72. IIMD results in oxidative stress (Russ et al., 2018) and WBHS decreases disuse atrophy-induced oxidative stress (Yoshihara et al., 2015). HSP72 is a negative regulator of proteolytic pathways (Senf et al., 2008; Yoshihara et al., 2015). Based on the pleiotropic properties of HSP72 that govern recovery of structure and function following injury, we postulate that WBHS induction of HSP72 attenuated IIMD and accelerated recovery of contractile function.
IIMD Treatment Studies
Previous studies have tested different strategies to treat IIMD and report varying degrees of success. Inhibition of myostatin (a negative regulator of muscle growth) and injection of muscle-derived stem cells both show promise. Inhibition of myostatin, via injection of suramin into the injury site enhances muscle regeneration and recovery of contractile function four weeks post-IIMD (Nozaki et al., 2008). These data suggest an important mechanism to enhance recovery of muscle function, however, suramin is not commercially available in the United States. Injection of muscle-derived stem cells into the injury site after IIMD is reported to enhance myogenesis, improve muscle quality, and accelerate recovery of contractile function (Kobayashi et al., 2016). Though promising, current methods limit the translation of this technique to injured humans. Other strategies, such as providing dietary fish oil to enhance membrane integrity before injury (Russ et al., 2018) and injection of platelet-rich plasma local to the IIMD site after injury to accelerate tissue repair and restore contractile function (Delos et al., 2014) do not hold as much promise. Other treatment strategies, such as icing (Puntel et al., 2011) and dietary supplementation with antioxidants (Kruger and Smith, 2012; Myburgh et al., 2012) indicate decreased overt muscle damage and oxidative stress, accelerated muscle regeneration, and an attenuated inflammatory response during the degeneration/inflammatory phase and regeneration phases. It is currently unknown if either of these treatment strategies accelerates recovery of contractile function following IIMD. Applying heat after IIMD may hold promise in humans. In a recent study (Kim et al., 2019) volunteers performed 300 maximal voluntary eccentric contractions in each leg, immediately after the injury one leg was heated to a skin temperature of 40oC and the other was the control; this was done again for each of the following four days. HSP72 mRNA levels increased equally in the quadriceps of heated-injured and non-heated-injured legs. Functionally, the heated leg was able to complete more work than the non-heated leg after 4-days of recovery. It is tempting to speculate that this heating strategy could accelerate recovery from IIMD in humans.
Limitations of the Study. While this study is the first to test if WBHS treatment prior to IIMD affects skeletal muscle contractile function and histological markers of muscle damage, the investigation was not without limitations. Major limitations of this study include the following: limited timepoints of contractile function assessment, qualitative assessment of immune cell invasion post-injury, and incomplete assessment of the tissue stress-response to IIMD. Specifically, contractile function was only measured at three time points. Earlier post-injury time points (e.g., immediately post-IIMD, 30-min, and 60-min post-IIMD) may help determine if WHBS attenuates edema. Additionally, direct histological identification of inflammatory cell types would add to the current early data by establishing the effect of WBHS on the timeline of extracellular and intracellular leukocyte invasion after IIMD. Finally, measurement of cytosolic Ca2+ concentration, oxidative stress markers, and cytokine and myogenic factor levels would add to our understanding of how WBHS accelerates recovery from IIMD.
Conclusion
This is the first study to report that WBHS prior to IIMD attenuates skeletal muscle damage and accelerates the rate of contractile function recovery. Furthermore, the 2-hour post-injury data suggest that WBHS treatment induces a protective mechanism to within skeletal muscle. Further studies are needed to uncover the differing response in the amount of edema that is observed between WBHS and NBT groups. The findings from this study also warrant future investigations into the mechanism(s) by which HSP activation provides this protection.
Conflicts of Interest
The authors declare they have no competing financial interest.
Acknowledgments
The authors would like to acknowledge S. Haley Brownlee and Nicholas J. Medlock for their skillful assistance with data collection.This work was supported in part by grants from Appalachian State University Office of Student Research and Appalachian State University Cratis D. Williams School of Graduate Studies.
References
Joshua S. Godwin1,2
1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608. 2Integrated Muscle Physiology Laboratory, Boone; North Carolina, 28608.
Charles F. Hodgman1,2
1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608. 2Integrated Muscle Physiology Laboratory, Boone; North Carolina, 28608.
Alan R. Needle1,3
1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608. 3Injury Neuromechanics Laboratory, Boone; North Carolina, 28608.
Kevin A. Zwetsloot1,2
1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608. 2Integrated Muscle Physiology Laboratory, Boone; North Carolina, 28608.
R. Andrew Shanely1,2
1Appalachian State University, Department of Health and Exercise Science; Boone, North Carolina, 28608. 2Integrated Muscle Physiology Laboratory, Boone; North Carolina, 28608.
Corresponding author:
R. Andrew Shanely
Email: shanelyra@appstate.edu

In a new window | Download PPT
Figure 1: In vivo torque-frequency relationship pre-impact induced muscle damage (IIMD) and post-IIMD. Whole-body heat shock (WBHS); normal body temperature (NBT). A) Pre-impact and 2-hours post-IIMD. B) Pre-impact and 2-days post-IIMD. C) Pre-impact and 5-days post-IIMD. *p < 0.05 within group, pre-IIMD vs post-IIMD. , # p < 0.05 between treatment groups, within time point. Data are means ± SEM.

In a new window | Download PPT
Figure 2: Submaximal and maximal in vivo torque and peak twitch/peak tetanic torque ratios. A) Submaximal 40 Hz stimulation frequency for both treatment groups at all time points. B) Maximal 250 Hz stimulation frequency for both treatment groups at all time points. C) Peak twitch (20 Hz) to maximal tetanic torque ratio (Pt/Po). The dotted line represents the average pre-impact induced muscle damage (IIMD) value for all treatment groups; normal body temperature (NBT), whole-body heat shock (WBHS). * p < 0.05 pre-IIMD vs post-IIMD, within group. Data are means ± SEM.

In a new window | Download PPT
Figure 3: Representative hematoxylin and eosin (H&E) stain, tibialis anterior myofibers, 20X magnification, scale bar represent 50 μm. A) uninjured, control. B) whole body heat shock (WBHS) 2-hour post-IIMD. C) Normal body temperature (NBT) 2-hours post-IIMD. D) WBHS 2-days post-IIMD. E) NBT 2-days post-IIMD. F) WBHS 5-days post-IIMD. G) NBT 5-days post-IIMD.

In a new window | Download PPT
Figure 4: Total number of damaged/regenerating fibers calculated as a percentage of the total number of fibers that demonstrated overt signs of injury. Control (Con), normal body temperature (NBT), whole body heat shock (WBHS). * p < 0.05 from control uninjured tibialis anterior muscle; # p < 0.05 between treatment groups, within time point; ‡ p < 0.05 within treatment group. Data are means ± SEM.

In a new window | Download PPT
Figure 5: Representative HSP72 expression (green) and nuclei (blue) in tibialis anterior myofibers, 10X magnification, scale bar represent 400 μm. Immunohistochemical staining was performed on A) normal body temperature (NBT), uninjured; B) 26-hours post-WBHS, uninjured; C) NBT, 2-hours post-IIMD; and D) 26-hours post-WBHS, 2-hours post-IIMD tibialis anterior myofibers.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9689 | 39 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA