Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
[Special Issue] COVID-19 and neurological symptoms: is the SARS-CoV-2 virus neurotropic?
Time:2020-11-20
Number:6078
David C Hess1, Elizabeth Rutkowski1, John Morgan1, Lynnette McCluskey2
Author Affiliations


- 1Department of Neurology, Medical College of Georgia at Augusta University.
- 2Neuroscience and Regenerative Medicine, Medical College of Georgia at Augusta University.
Condition Medicine 2020. 3(5): 241-245.
Abstract
Importance: The most notable symptoms of the Coronavirus Disease 2019 (COVID-19) pandemic are fever, cough, dyspnea, and in severe cases, adult respiratory distress syndrome (ARDS.) But neurological symptoms including confusion, stroke, and encephalopathy are reported, and anosmia and hypogeusia are also common indicating that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be neurotropic.
Observations: The SARS-Co-1 and 2 viruses bind to angiotensin converting enzyme 2 (ACE2), which is present on human brain endothelium and non-neuronal cells in the nasopharynx and lingual epithelium. However, SARS-CoV-1 and 2 do not bind rodent ACE2 avidly, which has required the generation of humanized ACE2 transgenic animal models of disease. Transgenic mouse models suggest that the SARS- CoV-1 and Middle East respiratory syndrome (MERS)-CoV are neurotropic and infect and damage the brain, including the cardiorespiratory centers in the medulla. The symptoms of anosmia and hypogeusia indicate a portal to the brain. The relationship between encephalitis lethargica and post encephalitis parkinsonism to the Spanish Flu (H1N1 influenza virus) is unclear but raises the question of long term neurological complications of pandemics.
Conclusions and Relevance: There is a concern that there may be long term neurological sequelae of infection with SARS-CoV-2. Registries and long term neurological follow up with longitudinal cohort studies of COVID19 positive patients are needed.
Keywords: COVID-19, SARS-CoV-1, SARS CoV-2, anosmia, hypogeusia
Abstract
Importance: The most notable symptoms of the Coronavirus Disease 2019 (COVID-19) pandemic are fever, cough, dyspnea, and in severe cases, adult respiratory distress syndrome (ARDS.) But neurological symptoms including confusion, stroke, and encephalopathy are reported, and anosmia and hypogeusia are also common indicating that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be neurotropic.
Observations: The SARS-Co-1 and 2 viruses bind to angiotensin converting enzyme 2 (ACE2), which is present on human brain endothelium and non-neuronal cells in the nasopharynx and lingual epithelium. However, SARS-CoV-1 and 2 do not bind rodent ACE2 avidly, which has required the generation of humanized ACE2 transgenic animal models of disease. Transgenic mouse models suggest that the SARS- CoV-1 and Middle East respiratory syndrome (MERS)-CoV are neurotropic and infect and damage the brain, including the cardiorespiratory centers in the medulla. The symptoms of anosmia and hypogeusia indicate a portal to the brain. The relationship between encephalitis lethargica and post encephalitis parkinsonism to the Spanish Flu (H1N1 influenza virus) is unclear but raises the question of long term neurological complications of pandemics.
Conclusions and Relevance: There is a concern that there may be long term neurological sequelae of infection with SARS-CoV-2. Registries and long term neurological follow up with longitudinal cohort studies of COVID19 positive patients are needed.
Keywords: COVID-19, SARS-CoV-1, SARS CoV-2, anosmia, hypogeusia
Introduction
There are increasing reports of neurological symptoms and complications of Coronavirus Disease 2019 (COVID-19) indicating that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus may be neurotropic.
Methods
We undertook a focused review of the neurological complications of COVID-19 using a search strategy and selection criteria: References for this Review were identified by searches of PubMed between 2000 and June 2020, and references from relevant articles. The search terms “COVID-19”, “SARS-CoV-1”, “SARS-CoV-2”, “anosmia”, “hypogeusia”, “MERS-CoV”, and “encephalitis lethargica” were used. We restricted selection to papers in English. The final reference list was generated on the basis of relevance to the topic of neurological complications of COVID19 covered in this Review.
Findings and discussion
A clue to early invasion and involvement of the nervous system are the early symptoms of anosmia and hypogeusia (Yan et al., 2020). These have been reported in otherwise asymptomatic patients. In a retrospective series of 214 patients (mean age 53; 41% male) from Wuhan, neurological symptoms occurred in 36.4% of patients (headache, dizziness, stroke, seizures). Neurological symptoms were more common in the severe as compared to the non-severe patients (45.5 vs. 30.2%). Strokes occurred in 5.6% of the severe group (Mao et al., 2020). In the Wuhan study, 11 (5%) had hyposomia and 12 (6%) hypogeusia.
In a study from Milan, Italy of hospitalized COVID-19 patients, of the 59 patients who were able to be interviewed (mean age 60 and 68% male), 20 (33.9%) reported at least one olfactory or taste disorder and 11 (18.6%) reported both. Females reported olfactory and taste disorders more frequently than males (10/19 [52.6%] vs. 10/40 [25%]; P = .036) (Giacomelli et al., 2020) In a cross sectional study using a survey of subjects who tested positive and negative for COVID-19, loss of smell was reported in 68% (40/59) and loss of taste in 71% (42/59) of COVID-19-positive subjects compared to 16% (33/203) and 17% respectively, (35/203) of COVID-19-negative subjects (p<.001) (Yan et al., 2020). On a severity scale, the loss of smell and taste were “profound” to “complete.” Seventy-four percent (28/38) of patients reported improvement of both smell and taste over a time period of a few weeks. In another study from Italy with a mean age of 56 and 48% in males, alteration of smell or taste was found in 64.4 % (130/202) of COVID-19 positive patients who were well enough to be managed at home (Spinato et al., 2020).
Anosmia is common after viral infections but is usually transient and results from destruction of the olfactory epithelium. Persistent anosmia and hypogeusia may result from involvement of the olfactory bulb, olfactory pathways, and brainstem with brain involvement (Fig 1). The finding of improvement in many of the patients suggest that only olfactory epithelium may be involved and not the olfactory bulb. However, anosmia that persisted for more than two years was reported in the SARS epidemic of 2003 (Hwang, 2006). Hypogesia may result from viral infection and inflammation in oropharyngeal taste buds and central pathways (Fig. 2) or could be secondary to olfactory deficits, which impact flavor perception (Henkin et al., 1975; Goodspeed et al., 1987; Graham et al., 1975; Wang et al., 2009).
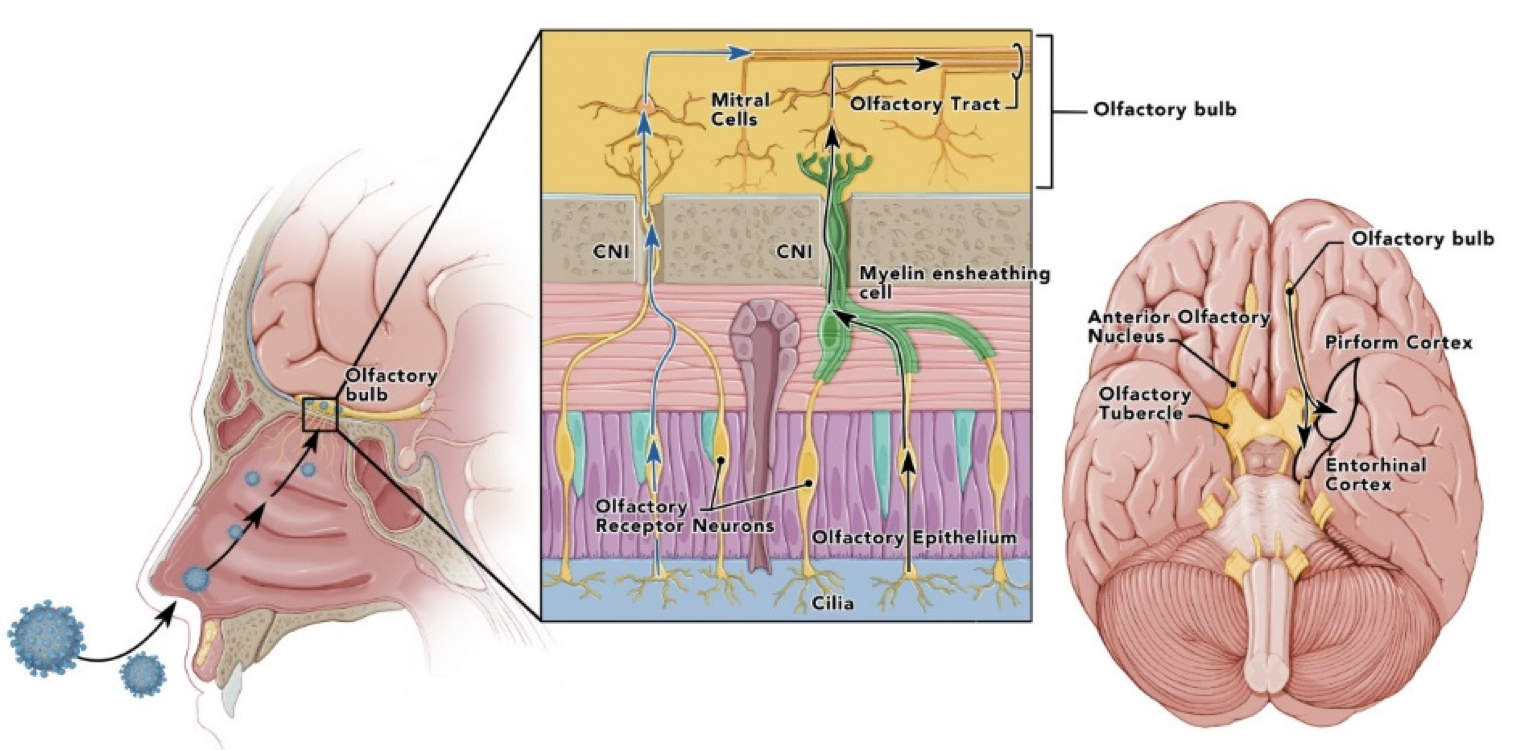
In a new window | Download PPT
Figure 1: Olfactory pathways with depiction of SARS-CoV-1 or 2 infecting nasopharynx with infection, or invasion of olfactory epithelium and spread up from the olfactory nerve to olfactory bulb and later into olfactory pathways including the pirirform cortex. It is not clear if virus spreads transneuronally or between axons and olfactory ensheathing cells or epithelial stem and supporting cells. (Cranial nerve = CN).
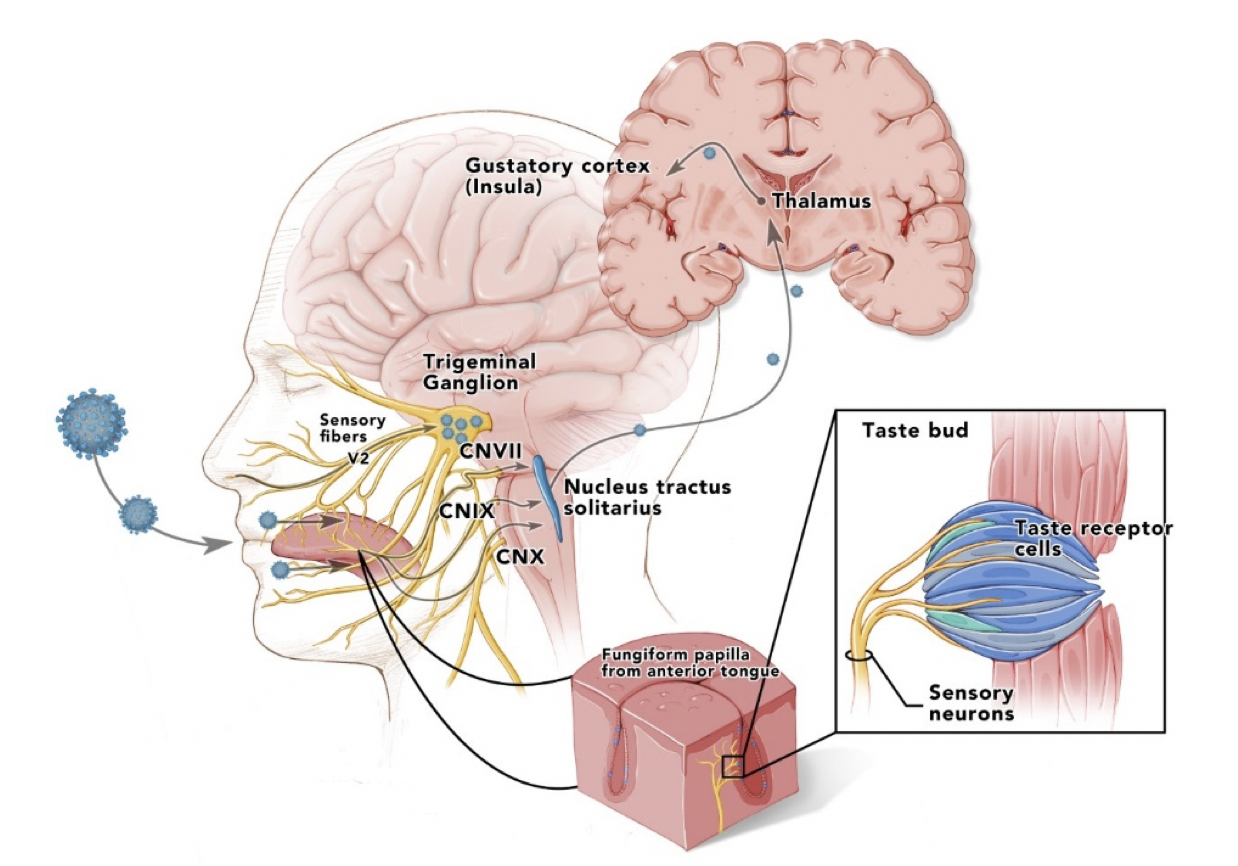
In a new window | Download PPT
Figure 2: Taste pathways showing infection of lingual fungiform, vallate papillae, and viral spread up the facial (chorda tympani), glossopharyngeal, and vagus nerves to ipsilateral nucleus solitarius in medulla, and then up to thalamus and cortex. Similarly, sensory fibers from nasopharynx could transport virus to the trigeminal ganglia anterior taste buds that might be more susceptible to inhaled virus than the rear vallate taste buds embedded in papillar moats. (Cranial nerve = CN).
SARS and Coronaviruses are neurotropic. According to the World Health Organization, the SARS epidemic that began in Guangdong province in southern China caused 8422 probable cases, 919 SARS-related deaths, and spread to 32 different countries or regions between November 2002 and August 2003 (Anon, n.d.). In the SARS 1 epidemic there were reports of serious neurological disease. One patient, a 39 year old intensivist who cared for SARS patients in southern China became infected and later developed worsening pulmonary problems and neurological deterioration with brain herniation, and on autopsy had evidence of aspergillosis in the lung and had positive PCR for SARS and positive immunostaining for SARS in the brain (Xu et al., 2005). A pregnant patient with seizures had SARS 1 virus in the cerebrospinal fluid (CSF) as detected by PCR (Lau et al., 2004). In an autopsy study of SARS patients, 8 of 8 patients with multi-organ failure had SARS viral genomic sequences detected in the brain by RT-PCR and in neurons by in situ hybridization. Six of 8 had neuronal degeneration and edema (Gu et al., 2005). Reports are now emerging of encephalitis with evidence of SARS-CoV- 2 in the CSF of COVID-19 patients (Moriguchi et al., 2020).
The SARS-CoV-2 spike protein binds to angiotensin converting enzyme 2 (ACE2) (Zhou et al., 2020). ACE2 is expressed in human alveolar epithelial cells and in the endothelial and smooth muscle cells in many organs including the brain (Hamming et al., 2004). Relevant to anosmia and hypogeusia, ACE2 is also expressed in non-neuronal cells in the olfactory epithelium and in the lingual epithelium (Brann et al., 2020; Xu et al., 2020). In the mouse, ACE2 is expressed in the olfactory epithelium, in non-taste and taste lingual epithelium (Shigemura et al., 2019), in neurons throughout the brain, in the brainstem in the cardiorespiratory center of the medulla in the magnocellular neurons of the paraventricular nucleus, the area postrema, the dorsal motor nucleus of the vagus, the nucleus of tractus solitarii , the rostroventrolateral medulla, and the nucleus ambiguous (Doobay et al., 2007).
One of the obstacles to SARS research is the lack of rodent models. SARS-CoV-1 and 2 bind to ACE2. Mouse and rat ACE2 do not avidly bind the spike protein and mice do not develop severe illness (McCray et al., 2007). A humanized ACE2 transgenic mouse develops severe respiratory and brain disease when infected with SARS (McCray et al., 2007). In this model, inoculation of SARS-CoV-1 into the nasopharynx led to the presence of the virus in the olfactory bulb within three days and then throughout the brain in the cerebrum, thalamus, and medulla (Netland et al., 2008). This was followed by cell loss in these structures without inflammation. However, there were increased cytokines including interleukin-6 (IL-6) in the brain. The lung pathology revealed changes compatible with aspiration leading to the conclusion that death was from brainstem involvement as the dorsal vagal complex was severely affected. Coronaviruses are known to spread trans-neuronally via both the olfactory and trigeminal nerves to the temporal cortex and brainstem within days (Perlman et al., 1990; Perlman and Netland, 2009). Some of the respiratory symptoms in COVID-19 may be related to altered respiratory drive from SARS-CoV-1 and 2 viruses infecting and injuring the medullary cardiorespiratory centers. An autopsy study of six COVID-19 patients from Munich Germany supports SARS-CoV-2 infecting and damaging the brainstem respiratory centers (von Weyhern et al., 2020). The investigators observed “localised perivascular and interstitial encephalitis with neuronal cell loss and axon degeneration in the dorsal motor nuclei of the vagus nerve, the trigeminal nerve, nucleus tractus solitarii, dorsal raphe nuclei, and fasciculus longitudinalis medialis” in all brains examined.
Middle Eastern Respiratory Syndrome caused by MERS-CoV and spread by dromedary camels appeared on the Saudi Arabian peninsula in 2012 with a severe respiratory syndrome including adult respiratory distress syndrome (ARDS), multi-organ failure, and death. The MERS-CoV binds to human dipeptidyl peptidase 4 (DPP4), which facilitates viral entry into the cell (Li et al., 2015). There were reports of three MERS-CoV patients all with pneumonia requiring mechanical ventilation with neurological symptoms including confusion, ataxia, focal left sided weakness, and coma (Harthi et al., 2015). Magnetic resonance imaging of the brain revealed severe white mater changes in the periventricular and subcortical white matter, including in the corpus callosum. There was no enhancement with gadolinium. CSF showed no pleocytosis and was negative for MERS-CoV PCR in the two patients tested.
Similar to the situation with SARS-CoV-1 and 2, MERS-CoV binds human DPP4, but it does not bind mouse DPP4 avidly and does not produce disease in mice. A transgenic mouse with humanized DPP4 is susceptible to MERS-CoV infection and develops severe infection in the lungs resembling human disease and brain invasion and involvement (Li et al., 2015). Neuronal lesions were most severe in the thalamus and brainstem. Virus antigen six days after infection was mostly located in the midbrain, thalamus, deep cerebral cortex, and CA2 region of the hippocampus.
Some of the neurological symptoms in the severely ill COVID-19 patients may not be related to direct viral invasion of the brain but instead cytokine release syndrome (CRS). This syndrome is associated with immune checkpoint inhibitors and chimeric antigen T cell (CAR-T cell) therapy in cancer (Shimabukuro-Vornhagen et al., 2018). CRS is associated with fever, headache, sepsis, and multiorgan failure that is related to macrophage secretion of cytokines such as IL-6. It is treated with IL-6 receptor antagonists such as tociluzimab, approved for rheumatoid arthritis. Immune effector-associated neurological symptoms (IBAN) are part of CRS and are characterized by confusion, expressive aphasia, seizures, and cerebral edema (Garcia Borrega et al., 2019).
The Spanish influenza of 1918 (H1N1) had an uncertain causal relationship to “encephalitis lethargica” that continues to be debated. Constantine Von Economo from Vienna described patients beginning in 1916 with flu-like symptoms, fever, somnolence, often with cranial neuropathies (Hoffman and Vilensky, 2017). There were “peaks” of encephalitis lethargica in 1920 and 1924. Some of these patients entered a “chronic form” months to years later of parkinsonism, often with “oculogyric crisis,” the “extinct volcanoes.” This postencephalitic parkinsonism is associated with substantial neuronal loss and gliosis in the brainstem, especially in the substantia nigra. There are neurofibrillary tangles in the brainstem, substantia nigra, and the globus pallidus that resemble those seen in progressive supranuclear palsy, but there are no Lewy bodies (Hoffman and Vilensky, 2017). One theory proposed by Poskanzer and Schwab (1963) from Harvard predicted that Parkinson’s disease resulted from a single etiology-subclinical infection from the etiologic agent of Economo’s disease between 1918 and 1920, and predicted the disease would precipitously decline by 1980. This theory has been disproven (Estupinan et al., 2013). That many suffered from neurological symptoms after the 1918 Spanish flu is illustrated by President Woodrow Wilson who suffered a bout of influenza at the Paris Peace Conference in 1919, 4 months prior to the right hemisphere stroke that disabled him (Barry, n.d.). His aides commented that he lost his focus and mental edge, gave into the demands of Prime Minister Clemenceau of France, and sacrificed his principles of self-determination to impose severe reparations on Germany that set the stage for the rise of Nazism and World War II.
Using reverse genetics, the reconstructed 1918 Spanish flu H1NI virus from RNA specimens of an infected Inuit woman buried in the Alaska permafrost, was intranasally inoculated into ferrets, infected the olfactory bulb and the brain. Infectious virus was found in the olfactory bulb and cerebrum at 1,3, and 5 days post inoculation and viral nucleoprotein in the brainstem and trigeminal ganglia at five days (De Wit et al., 2018). There was little inflammation but pro-inflammatory cytokines including IL-6 were upregulated in the olfactory bulb. These findings suggest the H1N1 virus that caused the 1918 Spanish flu was neurotropic.
Conclusions
There is a concern that some COVID-19 positive patients may suffer long term neurological sequelae. The 1918 Spanish flu’s causal association with encephalitis lethargica and parkinsonism is unclear and is still debated. The anosmia and hypogeusia in the COVID-19 pandemic may reflect viral destruction of olfactory epithelium, which regenerates and the patient should recover their sense of smell. But there remains the concern that the olfactory nerves and bulb and taste pathways are portals to the brain and that long term neurological complications are possible and of concern. Registries and prospective longitudinal cohort studies are needed to follow the COVID19 positive patients long-term for neurological complications. We have started a Georgia prospective longitudinal cohort study of COVID-19 positive patients, half of whom will be African American, in the Central Savannah Region Area of Georgia, to determine if there are long term neurological sequelae. We will employ standardized tests of smell and taste- the University of Pennsylvania Smell Identification Test (UPSIT) and the NIH Toolbox Gustation Test, and long term cognitive testing using the NIH Toolbox Cognition Battery.
Acknowledgement
Colby Zahn, M.S. Medical Illustrator, Department of Neurosurgery, Augusta University for Figures 1 and 2. David C Hess is funded by R01 NS099455; 1UO1NS113356; R01NS112511; 3R01NS112511-01A1S1 (Hess D).
Conflicts of interest
David C Hess MD is a consultant for Aruna Bio, Inc and receives fees for consultation and receives royalty payments from Athersys, Inc. Elizabeth Rutkowski and Lynnette McCluskey have no conflicts of interest/competing interests. John Morgan has the following:
Consultant: Acadia, Acorda, Adamas, Amneal, Eisai, Kyowa Kirin, Lundbeck, Parkinson’s Foundation.
Speaker: Acadia, Amneal, Kyowa Kirin, Parkinson’s Foundation.
References
David C Hess1
1Department of Neurology, Medical College of Georgia at Augusta University.
Elizabeth Rutkowski1
1Department of Neurology, Medical College of Georgia at Augusta University.
John Morgan1
1Department of Neurology, Medical College of Georgia at Augusta University.
Lynnette McCluskey2
2Neuroscience and Regenerative Medicine, Medical College of Georgia at Augusta University.
Corresponding author:
David C Hess
Email: dhess@augusta.edu

In a new window | Download PPT
Figure 1: Olfactory pathways with depiction of SARS-CoV-1 or 2 infecting nasopharynx with infection, or invasion of olfactory epithelium and spread up from the olfactory nerve to olfactory bulb and later into olfactory pathways including the pirirform cortex. It is not clear if virus spreads transneuronally or between axons and olfactory ensheathing cells or epithelial stem and supporting cells. (Cranial nerve = CN).

In a new window | Download PPT
Figure 2: Taste pathways showing infection of lingual fungiform, vallate papillae, and viral spread up the facial (chorda tympani), glossopharyngeal, and vagus nerves to ipsilateral nucleus solitarius in medulla, and then up to thalamus and cortex. Similarly, sensory fibers from nasopharynx could transport virus to the trigeminal ganglia anterior taste buds that might be more susceptible to inhaled virus than the rear vallate taste buds embedded in papillar moats. (Cranial nerve = CN).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6078 | 20 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA