Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Melatonin and cardioprotection: is it ready for clinical translation?
Time:2022-03-12
Number:7447
Rentia Lourens1, Aqeela Imamdin1, Sandrine Lecour1
Author Affiliations


- 1Cardioprotection Group, Hatter Institute for Cardiovascular Research in Africa, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Conditioning Medicine 2021. 4(6):271-279.
Abstract
Well-known to regulate the circadian rhythm, melatonin is a hormone that has multiple physiological properties, including cardioprotective properties. In the setting of ischemia-reperfusion injury, preclinical studies highlight the potential for melatonin to serve as an inexpensive, novel therapy against ischemia-reperfusion injury, as reduced infarct size and improved cardiac function are observed in the presence of melatonin. Disappointingly, clinical studies have not shown the same promise. In this review, we discuss factors that may explain the apparent inconsistencies between preclinical and clinical settings, including factors such as age, co-medication, co-morbidities, dosage, therapeutic window, and route of melatonin administration that may all affect its cardioprotective properties. A better understanding of these factors may improve translation to the clinical setting and determine whether melatonin can be considered as an effective cardioprotective therapy.
Keywords: Melatonin, Cardioprotection, Ischemia-reperfusion
Abstract
Well-known to regulate the circadian rhythm, melatonin is a hormone that has multiple physiological properties, including cardioprotective properties. In the setting of ischemia-reperfusion injury, preclinical studies highlight the potential for melatonin to serve as an inexpensive, novel therapy against ischemia-reperfusion injury, as reduced infarct size and improved cardiac function are observed in the presence of melatonin. Disappointingly, clinical studies have not shown the same promise. In this review, we discuss factors that may explain the apparent inconsistencies between preclinical and clinical settings, including factors such as age, co-medication, co-morbidities, dosage, therapeutic window, and route of melatonin administration that may all affect its cardioprotective properties. A better understanding of these factors may improve translation to the clinical setting and determine whether melatonin can be considered as an effective cardioprotective therapy.
Keywords: Melatonin, Cardioprotection, Ischemia-reperfusion
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of death worldwide, accounting for approximately 30% of deaths globally (Mensah et al. 2019; World Health Organization (WHO) 2017). Developing countries carry the highest burden of mortality from CVDs, with up to 80% of these deaths occurring in low and middle-income countries (Mensah et al. 2019). Various risk factors contribute to the development of CVDs, many of which can be modified through lifestyle adjustments, such as no smoking, exercise and dietary changes (World Health Organization, 2017). There is a pressing global need to identify novel, cost-effective ways to reduce this burden and recent data highlight melatonin as a potential candidate to protect against CVDs.
Melatonin (N-acetyl-5-methoxytryptamine) is a hormone well-known for its role in the regulation of the sleep/wake cycle and for its effectiveness as a treatment for jet lag and sleep-related disorders (Hardeland et al., 2006; Abdelgadir et al., 2018). It is mainly secreted by the pineal gland, a process regulated by the light/dark cycle, where light suppresses secretion of melatonin (Wurtman et al., 1963; Claustrat et al., 2005). Melatonin is also produced outside of the pineal gland and has a wide range of physiological properties including antioxidative, neuroprotective, anti-cancer, and anti-inflammatory properties (Hardeland et al., 2006; Shafabakhsh et al., 2019). Melatonin exerts most of its physiological effects after binding to receptors, including the melatonin 1 receptor (MT1) and melatonin 2 receptor (MT2) - both G protein-coupled receptors found at cell surfaces (Claustrat et al., 2005). Enzyme quinone reductase 2 has been identified as a cytosolic third melatonin receptor (Wu et al., 2018).
Over the past 20 years, there has been a large amount of preclinical and clinical studies supporting the role of melatonin in ischemic heart disease. Low levels of melatonin have been observed in patients with coronary heart disease (CHD) (Brugger et al., 1995) and a treatment with melatonin, given acutely or chronically, confers cardioprotection in rodent models of ischemia-reperfusion (I/R) injury (see review by Lochner et al., (2018)). Interestingly, melatonin is also present in food and there is preclinical evidence that melatonin given through the diet may protect against CVDs (Jiki et al., 2018; Salehi et al., 2019; Sangsopha et al., 2020). As such, melatonin treatment has the potential to become a cost-effective therapy against CVDs. Unfortunately, clinical trials that have tested melatonin as a treatment to limit I/R injury have not yet led to the expected outcomes (Ekelof et al., 2016; Dominguez-Rodriguez et al., 2017a; Dominguez-Rodriguez et al., 2017b; Ekeloef et al. 2017). Here, we review both preclinical and clinical research studies that assessed melatonin as a possible cardioprotective therapy against I/R injury and we discuss the translational challenges that may explain the disappointing outcomes with the use of melatonin as a cardioprotective therapy in humans to date.
Cardioprotective properties of melatonin – preclinical studies
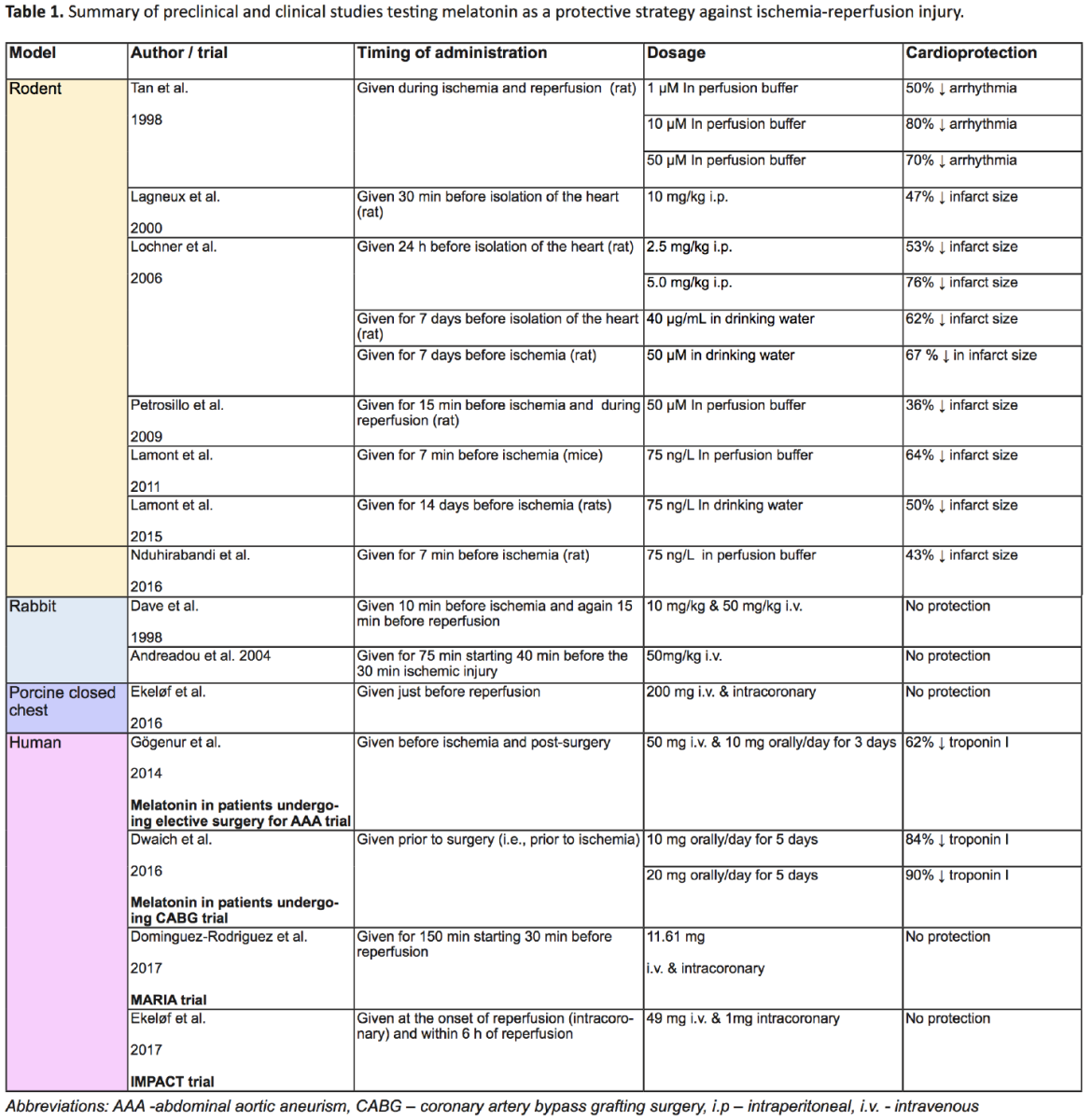
Various animal studies have shown a promising outcome when testing melatonin as a cardioprotective therapy (see Table 1).
In 1998, Tan and colleagues (1998) were the first to describe the cardioprotective effect of melatonin (Tan et al., 1998). Perfusion of melatonin at a concentration of 10 μM in ex-vivo rat hearts reduced arrhythmias associated with I/R injury when administered during the reperfusion phase (Tan et al., 1998). Similarly, intraperitoneal administration of 10 mg/kg melatonin prior to an ex vivo I/R injury reduced both infarct size and arrhythmias (Lagneux et al., 2000). Interestingly, reduction of the infarct size was observed after both short- and long-term treatment with melatonin given prior to I/R injury (Lochner et al., 2006). In this later study, the short-term arm of the study with melatonin perfused to the isolated heart, included 3 groups; (1) one group treated with melatonin both before ischemia and during reperfusion (50 μM), (2) one group treated before ischemia only, and (3) one group treated during reperfusion only using the isolated heart system (Lochner et al., 2006). The long-term arm included a group receiving melatonin treatment orally (40μg/L) prior to ischemia (Lochner et al., 2006). All treated groups showed significant reduction in infarct size compared to the control group, except for the short-term group that received melatonin just before the onset of ischemia (Lochner et al., 2006). The protection afforded by the long-term treatment strategy lasted up to four days after the end of the treatment (Lochner et al., 2006). This protective effect was abolished by co-treatment with luzindole, a melatonin receptor blocker, thus suggesting that melatonin exerts its protective effects through the melatonin MT1 and MT2 receptors (Lochner et al., 2006).
Several other studies using the isolated rat heart model confirmed a reduction in infarct size following administration of melatonin (Lamont et al., 2011; Nduhirabandi et al., 2016) and a similar cardioprotective effect of melatonin was also confirmed in an in vivo model of I/R injury (Lamont et al., 2015).
In rodents, the cardioprotective effect of a very low concentration of melatonin (75 ng/L) added to the drinking water (similar to the concentration present in wine) was found to be mediated through the activation of the survivor activating factor enhancement (SAFE) pathway, which involves the activation of tumour necrosis factor alpha (TNFα) and signal transducer and activator of transcription 3 (STAT3) (Lamont et al., 2011; Lamont et al., 2015). The activation of the SAFE pathway by melatonin is likely mediated by the toll-like receptor 4 (TLR4), which is known to activate STAT3 via TNFα (Nduhirabandi et al., 2016). Administration of melatonin prior to ischemia did indeed reduce the infarct size in rats, but this effect was lost in the presence of TLR4 inhibitors (Nduhirabandi et al., 2016).
A rodent model of I/R injury showed that melatonin prevented the opening of the mitochondrial permeability transition pore (MPTP) and the release of cytochrome c in I/R injury, an effect that was associated with a better recovery (Petrosillo et al., 2006). Cardiolipin is a phospholipid component of the inner mitochondrial membrane that is easily targeted by reactive oxygen species (ROS) (Petrosillo et al., 2006), and it is thought that peroxidized cardiolipin possibly triggers the opening of MPTP to release cytochrome c, a pro-apoptotic factor (Petrosillo et al., 2006; Petrosillo et al., 2009). Melatonin is known to have antioxidative effects and it prevents the oxidation of cardiolipin, further supporting its beneficial role in cardioprotection (Petrosillo et al., 2009).
Cardioprotective properties of melatonin –clinical studies
Observational studies
The cardioprotective effect of melatonin in preclinical studies was first supported by observational studies conducted in humans. Lower serum melatonin levels were observed in patients suffering from acute myocardial infarction (MI) compared to controls (Dominguez-Rodriguez et al., 2012). An inverse correlation between the severity of symptoms and melatonin levels was detected, with lower serum melatonin levels correlating with adverse symptoms (Dominguez-Rodriguez et al., 2012). In particular, patients with pathological left ventricular (LV) remodeling had lower levels of melatonin (Dominguez-Rodriguez et al., 2012). Based on the preclinical findings and these observational studies, a few clinical trials have been designed to explore the cardioprotective effect of melatonin against ischemia-reperfusion injuries (Table 1).
MARIA trial
The Melatonin Adjunct in the acute myocaRdial Infarction treated with Angioplasty (MARIA) trial was the first study designed to test the effect of melatonin in human ST-elevation myocardial infarction (STEMI) patients (Dominguez-Rodriguez et al., 2007). The treatment protocol consisted of an intravenous administration of 100 μM melatonin in first time MI patients for 150 minutes starting 30 minutes before percutaneous revascularization (Dominguez-Rodriguez et al., 2007). Its effects were assessed by measuring the cumulative release of alpha-hydroxybutyrate dehydrogenase and peak value of troponin l (Dominguez-Rodriguez et al., 2007). The trial ran from 2013 to 2015, but the results of the study were unable to provide sufficient evidence that melatonin can attenuate the effects of MI, or at least justify clinical use of melatonin in MI patients (Dominguez-Rodriguez et al., 2017a; Dominguez-Rodriguez et al., 2017b). When patients were classified into three groups based on the time of presentation after symptom onset, the data suggested that melatonin may protect the patients who presented early, but may be detrimental in patients who presented late (Dominguez-Rodriguez et al., 2017a; Dominguez-Rodriguez et al., 2017b). No effect of melatonin was observed in patients in the middle group.
IMPACT trial
The Intracoronary and systemic Melatonin to Patients with Acute myocardial infarCTion (IMPACT) trial was another clinical study aiming to explore the potential of melatonin in MI patients. The purpose of this trial was to investigate if melatonin, potentially through its antioxidative properties, may help alleviate reperfusion injury in STEMI patients following primary percutaneous coronary intervention (pPCI) (Halladin et al., 2014; Ekeloef et al. 2017). Patients received 50 mg of melatonin, with 1 mg given as an intracoronary bolus 1 minute after the onset of reperfusion and 49 mg administrated intravenously in the 6 hours immediately following pPCI (Halladin et al., 2014). Two groups of 23 patients who received pPCI within 6 hours of symptom onset were included in this study, and the myocardial salvage index (MSI) scale was used to estimate infarct size (Halladin et al., 2014). The results of this study were disappointing, with no differences in I/R injury or inflammatory markers observed between the melatonin and placebo groups (Ekeloef et al. 2017).
Melatonin in patients undergoing coronary artery bypass grafting surgery
Another study was performed to determine whether melatonin could alleviate cardiac injury associated with coronary artery bypass grafting surgery (CABG) (Dwaich et al., 2016). Forty-five patients were included in the study (Dwaich et al., 2016). Melatonin was administered orally (10 or 20mg) for 5 days before surgery. Melatonin treatment was associated with a reduction of troponin-I release, an increased ejection fraction, and lower inducible nitric oxide synthase. The protection appeared to be related to dosage as the 20 mg dosage group showed better protection than the 10 mg dosage group (Dwaich et al., 2016).
Melatonin in patients undergoing elective surgery for abdominal aortic aneurism
I/R injury commonly occurs in patients undergoing abdominal aortic aneurism (AAA) surgery and melatonin was tested in this setting (Gogenur et al., 2014). The study included 50 participants: 26 participants were given melatonin and the remaining pariticpants received a placebo. Melatonin was administered at 50 mg intravenously before ischemia, and 10 mg orally for three days following surgery. Troponin-I and ST-segment deviations were analyzed, and patients receiving melatonin had significantly less damage than the control group (more than 60% reduction in troponin-I), thus suggesting that, in this case, melatonin was protective (Gogenur et al., 2014).
In summary, most clinical intervention trial results fall short of the initial promise shown by preclinical studies. The lack of translation from preclinical to clinical studies is complex and may be the result of the influence of a number of factors.
Factors that may explain the lack of translation in clinical studies
A closer look at the methodology of published studies exploring the cardioprotective effect of melatonin highlights the lack of rigor in the design of most studies, with preclinical studies poorly mimicking the clinical setting, and vice versa, clinical studies that may have been designed without taking into account the knowledge that can be obtain from preclinical studies. Multiple factors may alter the cardioprotective effect of melatonin such as age, co-morbidities, and co-medication.
Age
Factors contributing to poor translation from rodent models to the clinical setting include poor consideration of age in animal studies (Jackson et al., 2017). Age plays a role in most diseases, and poor choice of rodent age in studies leads to somewhat incomparable results, as diseases and drug mechanisms vary with age. Rodents used in most biomedical studies are between 8 and 12 weeks of age, an age where physiological changes and development still take place and are not always representative of human adult physiology, especially in the field of acute MI (Jackson et al., 2017). To improve translation of biomedical research, the age of the animals should be in line with the age where the disease/drug being studied is most relevant and the limitation of the age range should clearly be discussed in research articles for future studies and reproducibility of the results (Lecour et al., 2021a).
Most importantly, melatonin production varies according to age (Touitou, 2001). Serum melatonin levels peak from age one to three, after which it declines. It is estimated that serum melatonin levels in teenagers declines by roughly 80% and continues to decline with age (Touitou, 2001). A similar decrease in melatonin levels is seen in aging rodents (Selmaoui and Touitou, 1999). It is therefore important to interpret with caution the results testing melatonin in young animals as the endogenous melatonin production in these animals differs from older animals.
Co-morbidities
Variation in melatonin production and levels are associated with some diseases, including diabetes, kidney, and liver disease (Espino et al., 2011; Ishigaki et al., 2016). This means that administration of melatonin in patients with these co-morbidities is complex and should be carefully considered in translational research.
Circadian rhythms relate to metabolic disorders – an irregular circadian rhythm may correlate with glucose intolerance, thus circadian rhythms and melatonin production may have an effect on diabetes (Espino et al., 2011). Indeed, it is thought that melatonin may suppress β-cell activity (as it appears that β-cells have melatonin receptors), reducing insulin levels, thus indicating an inverse relationship between melatonin and insulin levels (Espino et al., 2011). Type 1 and type 2 diabetes appear to have differential correlation with melatonin levels in rats, with type 2 presenting with decreased melatonin levels, and type 1 with increased melatonin levels (Peschke et al., 2015). The interplay between insulin and melatonin may be part of a feedback loop, each affecting the expression of the other, thus rendering diabetes a confounder when it comes to melatonin levels in the body (Peschke et al., 2015).
In addition, mutations in melatonin receptors are closely associated with certain co-morbidities, with mutations in MT2, for example, affecting insulin and glycogen secretion/synthesis (Mussig et al., 2010; Espino et al., 2011). Mutations in MT1 are associated with insulin resistance in mice, suggesting that melatonin plays a role in the development of type 2 diabetes (Espino et al., 2011). Mutations in the melatonin receptor variant MTNR1B is associated with changes in the circadian rhythm, melatonin secretion, and an increased risk of developing type 2 diabetes (Lane et al., 2016). An inverse correlation between melatonin levels and type 2 diabetes risk has been observed, thereby suggesting that people with lower serum melatonin levels have a higher risk of developing type 2 diabetes (McMullan et al., 2013). Although treatment with melatonin protects against I/R injury in preclinical models of diabetes by mechanisms that include a reduction in ROS formation and the activation of sirtuins-1, its effectiveness in diabetic patients remains to be confirmed (see review (Andreadou et al., 2021).
In kidney diseases, the dysregulation of the renin-angiotensin system (RAS) plays a major role in renal damage Ishigaki et al., 2016). Activation of RAS is related to the circadian rhythm, which contributes to diurnal variation in blood pressure (Kobori et al., 2007; Ishigaki et al., 2016). The damage caused by RAS activity is initiated (in part) by release of ROS and inhibition of nitric oxide (Kobori et al., 2007; Ishigaki et al., 2016). Melatonin, which is associated with the circadian rhythm and known as an antioxidant, was shown to be associated with chronic kidney disease (Ishigaki et al., 2016). Low night-time serum melatonin levels are associated with night-time activation of the renin-angiotensin-system (RAS) in kidney disease and renal damage (Ishigaki et al., 2016).
Similarly, melatonin has been shown to protect against acute liver injury induced by carbon tetrachloride (CCl4) in rats (Ohta et al., 2000). This protection is thought to be largely mediated by the strong antioxidative properties of melatonin (Ohta et al., 2000). This benefit against liver damage is further supported by findings that melatonin can alleviate damage from bile duct ligation-induced liver injury (Sheen et al., 2016).
Melatonin is involved in a variety of diseases, and its regulation is also affected by various diseases, including metabolic diseases. To be able to successfully translate it to the clinical setting, the effect of these co-morbidities on the cardioprotective effect of melatonin should be carefully studied in the preclinical setting prior to embarking on a clinical trial.
Co-medication
Drug interaction often occurs when two or more drugs that are metabolized by (or act on) the same enzyme are taken simultaneously, leading to the disruption of metabolism of one or more of these drugs (Papagiannidou et al., 2014). Co-medications that might interfere with the metabolism of melatonin should be considered in the translation of melatonin treatment to the clinical setting.
Melatonin is mainly metabolised by CYP1A2, a microsomal enzyme involved in the metabolism of a variety of drugs (Papagiannidou et al., 2014). Drugs that inhibit CYP1A2 leading to higher serum melatonin levels include fluvoxamine (an antidepressant), diazepam (a benzodiazepine drug used to treat anxiety), 17α-ethinyloestradiol (a product present in oral contraceptives), 5-methoxypsoralen (drug used to treat psoriasis), and caffeine (Papagiannidou et al., 2014). Tobacco, on the other hand, contains polycyclic aromatic hydrocarbons, products that upregulate CYP1A2, leading to rapid melatonin metabolism and decreased serum levels. Of the drugs studied, 5-methoxypsoralen was found to have the most adverse effect and it was found that 17α-ethinyloestradiol could be beneficial in individuals with low serum melatonin levels, since it could prolong the hormonal effect of melatonin (Papagiannidou et al., 2014).
Possible interaction between melatonin and warfarin (an anticoagulant) have recently been noted (Ashy and Krishna, 2016). Warfarin is prescribed to many CVD patients to prevent formation of blood clots and there is evidence that melatonin might increase its anticoagulation capacity, thus increasing the risk of bleeding (Ashy and Krishna, 2016). These medications can therefore easily present confounding factors in melatonin-related research, and these should be taken into careful consideration to ensure successful translation.
Choice of animal model
Translation of biomedical research from animal models to the clinical settings are lacking in part due to poor experimental design in animal models chosen to mimic clinical settings (Jackson et al., 2017; Lecour et al., 2021a; Lecour et al., 2021b). Rodents are most commonly used in animal models, but these animal models are not necessarily representative of humans, particularly when considering that rodents are nocturnal animals and that experimental studies are performed during their time of sleep.
Although rodent studies present melatonin as a promising cardioprotective therapy, this effect failed to be reported in other animal species. A porcine model study by Ekeløf et al. (2016) investigating the effect of melatonin on myocardial I/R injury produced disappointing results (Ekelof et al., 2016). The study involved 20 female pigs, randomized into experimental and control groups. pPCI was performed and 200 mg of melatonin (or isotonic saline in the control group) was administered by intravenous and intracoronary routes, 5 minutes before the onset of reperfusion. I/R injury was assessed using the MSI and by measurement of troponin T, but no significant differences were found between the control and experimental groups (Ekelof et al., 2016). This finding was echoed in a similar study on rabbits (Dave et al., 1998). Rabbits were treated with 10 mg/kg or 50 mg/kg melatonin 10 minutes before the onset of ischemia and again 15 minutes before the onset of reperfusion. No differences were observed between the control and experimental groups (Dave et al., 1998). Similar findings were later confirmed by Andreadou et al. (2004).
There has been inconsistency across animal models, with results suggesting that melatonin has cardioprotective potential in rat models, but not in porcine or rabbit models, which that have more similar circadian rhythms to humans (Dave et al., 1998; Ekeloef et al. 2017). In fact, the circadian rhythm of pigs appears to be close to humans, indicating that a porcine model may be a better representation of humans in melatonin-related research (Engert et al., 2016). The porcine trial protocol mimicked that of the MARIA trial but was started after the MARIA trial had already begun (Ekelof et al., 2016; Dominguez-Rodriguez et al., 2017b). The expected outcome of the clinical trial may have been foretold by that of the porcine trial if they had followed sequentially.
Time, route, and duration of administration
Timing, route, and duration of melatonin administration are all factors that could greatly affect plasma concentration of melatonin and should therefore be optimized and carefully considered in translation (Touitou, 2001).
Time of administration
Melatonin given as a cardioprotective agent in preclinical studies is typically administered before I/R injury, during I/R injury, or after I/R injury. In animal studies, melatonin administration before onset of ischemia has been shown to be more protective than its administration during I/R injury (after ischemia, before reperfusion), thus indicating that its efficacy may be dependent upon the time of administration relative to injury (Tan et al., 1998). Scant studies suggest melatonin treatment could either be given before ischemia and during reperfusion, or only during reperfusion, but administration before ischemia and during reperfusion proved to be more protective than during reperfusion alone (Lochner et al., 2006). As melatonin is tightly linked to circadian rhythms, the timing of administration during the day may also affect its activity as a cardioprotective agent, however levels of melatonin administered exogenously at different times along the day is an avenue of research yet to be explored.
Route of administration
Melatonin can be given via multiple routes of administration including intravenously, intraperitoneally, and orally. There is limited information on which route is most effective, though oral administration is considered to be generally more effective than intraperitoneal administration (Kireev et al., 2013). A study on the pharmacokinetics of orally and intravenously administered melatonin found that intravenous melatonin led to much higher serum concentrations than oral melatonin (Andersen et al., 2016). In this study, both groups received 10 mg of melatonin, which led to maximum plasma concentrations of 389,875 pg/ml and 3550 pg/ml in the intravenous group and oral group, respectively (Andersen et al., 2016). These results show that the route of administration largely determine serum melatonin concentrations. However, such high concentrations, as seen with intravenous administration, are not necessarily more beneficial and are largely extra-physiological values. Most concerning is the fact that the route of administration chosen in clinical study designs does not appear to be based on the findings of preclinical studies. More rigorous experiments are needed to delineate the optimal route of administration of melatonin for cardioprotective studies.
Duration of the treatment
Duration of melatonin administration refers to long-term and short-term treatments. Both long-term and short-term administration of melatonin have shown promise (Lochner et al., 2006). In rodents, long-term administration of melatonin can lead to lasting cardioprotective effects of up to 4 days, which is not the case with a short term treatment (Lochner et al., 2006). It has been suggested though, that to optimise short-term treatment, melatonin should be administered throughout the entire reperfusion period (Duncker and Verdouw, 2001). There is still much research to be done to determine whether long- or short-term treatment with melatonin is more beneficial for cardioprotection.
Dosage of exogenous melatonin and modulation of endogenous melatonin concentration
Dosage determines serum melatonin levels to a large extent, which like most other hormone levels in the body, needs to be carefully controlled as too little or too much may lead to physiological disturbances (Guardiola-Lemaitre, 1997; Touitou, 2001; Espino et al., 2011). Current literature shows a gap when it comes to dose response studies in animals to ascertain melatonin’s efficacy and physiological soundness. An excess of melatonin might have a negative effect on glucose metabolism and insulin sensitivity, which is why it is important to carefully control the dosage (Guardiola-Lemaitre, 1997; Zanquetta et al., 2003; Espino et al., 2011). At low dosages, melatonin failed to protect, and at excessive dosages, the protective effect may be diminished (Tan et al., 1998).
In children with heart failure, melatonin levels were increased and correlated to the severity of heart failure (Wu et al., 2018). This finding is surprising, as melatonin is considered to be cardioprotective, and most studies have revealed that lower melatonin levels are observed in patients with CVDs. It is possible that the melatonin levels rise with severity as a compensatory mechanism, though further research is still needed to clarify this explanation (Wu et al., 2018). These findings, however, emphasize that dosage of melatonin should be carefully controlled, as it is still unknown whether high melatonin levels can be detrimental. It should also be noted that administration of the therapeutic dose of melatonin commonly used for jet-lag leads to extra-physiological serum concentrations of melatonin (Andersen et al., 2016). However, it may not be necessary to supplement melatonin chemically – dosages of which can account for more than a 100 - fold increase above physiological melatonin levels (Dwaich et al., 2016). Beneficial levels of melatonin can easily be attained through the diet, which raises serum levels of melatonin to physiological values (Sae-Teaw et al., 2013; Jiki et al., 2018; Varoni et al., 2018). Various foods increase serum melatonin levels, including walnuts, vegetables, fruit, oils, coffee, tea, beer, wine, and even some meats (Reiter et al., 2005; Tan et al., 2014). Walnuts, known to reduce the risk of CVDs, contain 3.5 ± 1.0 ng/g melatonin (Reiter et al., 2005). Pineapples, oranges, and bananas consumption have resulted in significant increase in serum melatonin levels, and contain on average 302 pg, 150 pg, and 8.9 pg of melatonin per gram of wet fruit, respectively (Sae-Teaw et al., 2013). Melatonin is found in wine at concentrations between 50 ng/L to 200 ng/L and is thought to contribute to the cardioprotective effect of moderate and regular consumption of wine (Lamont et al., 2011; Lamont et al., 2015). The melatonin concentrations found in food are sufficient to significantly increase serum melatonin levels within the physiological range one hour after ingestion (Sae-Teaw et al., 2013; Varoni et al., 2018).
Time of day
Melatonin production is largely related to the circadian rhythm with light stimuli suppressing its production (Touitou, 2001). Serum levels of melatonin fluctuate throughout the day, with highest production at night (Touitou, 2001). Timing of melatonin administration matters as inappropriate timing can lead to supraphysiological melatonin levels and negatively affect receptor activity (Espino et al., 2011). It is therefore important, in translation, to carefully consider the time of day at which melatonin treatment is administered, measured, and most effective.
Metabolism of melatonin
As previously mentioned, melatonin is mainly metabolized by the CYP1A2 enzyme of the cytochrome P450 family (Skene et al., 2001; Papagiannidou et al., 2014). Melatonin has a short half-life, estimated to be approximately an hour, but there seems to be great variability in this among individuals (Papagiannidou et al., 2014). In individuals with poor CYP1A2 metabolism, plasma melatonin is remarkably high, and the pharmacological effect of melatonin would likely be muted (Braam et al., 2010; Papagiannidou et al., 2014). High serum levels of melatonin are therefore indicative of slow metabolism and explains why the desired treatment effect is not seen (Lockley et al., 2000; Braam et al., 2010; Papagiannidou et al., 2014). When investigating the protective effect of melatonin, it is important to keep in mind that the rate of metabolism of melatonin between individuals varies, and that poor melatonin metabolism may affect the expected outcomes.
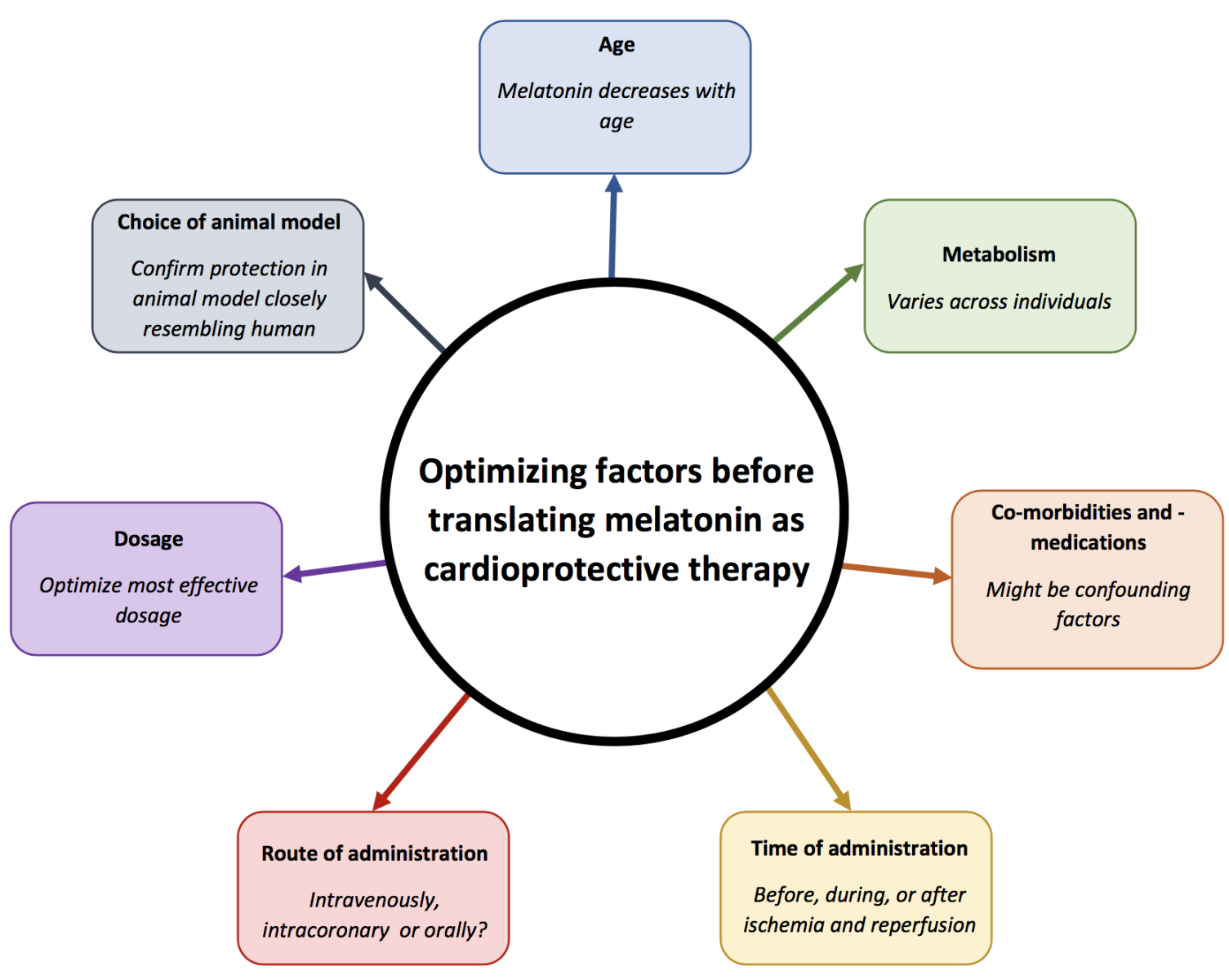
In summary, a large number of confounding factors may influence the cardioprotective effect of melatonin. Some of these factors - for instance time and route of administration - may very well contribute to explain the disappointing outcomes of clinical trial testing the cardioprotective effect of melatonin such as the MARIA and IMPACT trials. The dosage, route, and time of administration are particularly important factors to optimize before melatonin can be successfully tested in the clinical setting. Further research should therefore be conducted on these factors, to determine the optimal route of administration. It seems that melatonin administration before onset of ischemia may be somewhat more protective than after onset. Though melatonin cannot be administered before ischemia in patients suffering from MI, it may have potential to be used as a preventative measure in CVD patients at risk for MI. Optimization of melatonin administration (Figure. 1) may help to maximize cardioprotection and thus reduce the burden of CVDs. Furthermore, optimization of melatonin via the diet intake should be considered as a safe and inexpensive approach to limit I/R injury. Research on the use of melatonin as a cardioprotective strategy still holds much promise and should continue, bearing all these factors in mind with rigorous preclinical investigation prior to clinical testing.
In a new window | Download PPT
Figure 1: Key factors that may affect the cardioprotective effect of melatonin.
Conclusion
CVDs are a growing problem, in high-, middle-, and low-income countries. It is of great importance to find cost-effective strategies to lower the burden. Melatonin, which is present in many food types, has shown promise as a cardioprotective strategy, but has proved to be challenging to translate to the clinical setting. There are many factors that contribute to the lack of translation. Careful consideration and rigorous optimization of these factors, which include dosage, time, and route of administration, among others, may smooth the course to successful translation. Considering the burden of CVDs, the cost-effectiveness and safety of melatonin, and the promise shown by preclinical studies, it seems worthwhile to continue doing research to fine-tune the use of melatonin as a cardioprotective agent.
Ethics approval and consent to participate
This is a review paper that does not include any direct patient or animal data. Therefore, the ethics approval or consent is not applicable to this case.
Competing interests
The author(s) declare no competing interests.
Acknowledgements
A.I. was supported by a Fellowship from the University of Cape Town. R.L. was supported by the National Research Foundation and the University of Cape Town. S.L. was supported by the University of Cape Town, the National Research Foundation, Winetech.
References
Rentia Lourens1
1Cardioprotection Group, Hatter Institute for Cardiovascular Research in Africa, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Aqeela Imamdin1
1Cardioprotection Group, Hatter Institute for Cardiovascular Research in Africa, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Sandrine Lecour1
1Cardioprotection Group, Hatter Institute for Cardiovascular Research in Africa, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa.
Corresponding author:
Sandrine Lecour
Email: Sandrine.lecour@uct.ac.za

In a new window | Download PPT
Figure 1: Key factors that may affect the cardioprotective effect of melatonin.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7447 | 7 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA