International bi-monthly journal of cell signaling, tissue protection, and translational research.
Circadian Effects on Clinical Outcomes following Acute Myocardial Infarction, Cardiac Procedures, and Surgery
Junsuk Ko1, Ching-Hui Sia2,3, Derek J Hausenloy1,3,4,5,6
Author Affiliations


- 1Duke-NUS Medical School, National University of Singapore, Singapore.
- 2Department of Cardiology, National University Heart Centre Singapore, Singapore.
- 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 4National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 5The Hatter Cardiovascular Institute, University College London, London, United Kingdom.
- 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Abstract
Circadian rhythm is a potentially important regulator of the (patho)physiology governing gene transcription and metabolism. Data from basic science studies suggest that critical cellular processes, such as oxidative stress and regeneration after ischemia and reperfusion injury (IRI), are under the control of circadian rhythm. This has implications for patients presenting with acute myocardial infarction (AMI) given that IRI is a key determinant of clinical outcomes in this patient group. However, data interpretation in the field of circadian rhythm research has been challenging due the the presence of multiple biological and non-biological factors. Circadian rhythm is affected by non-biological factors such as healthcare workers’ fatigue, limited resources, and significant delay in providing critical care at night. This review aims to evaluate current evidence of the impact of circadian rhythm on clinical outcomes in AMI patients and in patients undergoing cardiac surgery.
Keywords: Circadian Rhythm, Cardiac Surgery, Myocardial Infarction, Percutaneous Intervention, Coronary Artery Bypass Graft, Aortic Valve Replacement
Abstract
Circadian rhythm is a potentially important regulator of the (patho)physiology governing gene transcription and metabolism. Data from basic science studies suggest that critical cellular processes, such as oxidative stress and regeneration after ischemia and reperfusion injury (IRI), are under the control of circadian rhythm. This has implications for patients presenting with acute myocardial infarction (AMI) given that IRI is a key determinant of clinical outcomes in this patient group. However, data interpretation in the field of circadian rhythm research has been challenging due the the presence of multiple biological and non-biological factors. Circadian rhythm is affected by non-biological factors such as healthcare workers’ fatigue, limited resources, and significant delay in providing critical care at night. This review aims to evaluate current evidence of the impact of circadian rhythm on clinical outcomes in AMI patients and in patients undergoing cardiac surgery.
Keywords: Circadian Rhythm, Cardiac Surgery, Myocardial Infarction, Percutaneous Intervention, Coronary Artery Bypass Graft, Aortic Valve Replacement
Introduction
Circadian rhythm is an endogenous oscillating pattern of molecular and physiological activities with a near-24-hour period that allows organisms to adapt to external stimuli and the environment (Patke et al., 2020). The circadian rhythm is regulated by circadian clocks. There is a central clock involving neurons residing in the suprachiasmatic nucleus of the hypothalamus and a peripheral clock that is present in other tissues (Crnko et al., 2019). Light transmitted from the retina to the suprachiasmatic nucleus is an important biological cue that not only regulates the central clock but also synchronizes the peripheral clock via hormones and neurotransmitters (Cajochen et al., 2003). The circadian rhythm is a potentially important regulator of cardiovascular physiology and pathogenesis (For further reading see Hausenloy and Yellon, 2017; Thosar et al., 2018; Slomski, 2019; Dong et al., 2020; Rana et al., 2020; Lecour et al., 2021) as multiple studies suggest that peripheral clock gene expression displays a circadian pattern in cardiac cells, including cardiomyocytes (Beesley et al., 2016), endothelial (Takeda et al., 2007), vascular smooth muscle (Lin et al., 2014), and stem cells (Du Pré et al., 2017). Moreover, blood pressure is under the influence of the circadian rhythm (Xu et al., 2019; Cortés-Ríos and Rodriguez-Fernandez, 2021; Zhang et al., 2021). Despite the well-documented demonstration of circadian patterns for cellular activities and gene transcription in isolated cardiovascular cells in vitro, the exact impact of circadian rhythm in major cardiac diseases and prognosis after cardiac interventions is still unclear. This controversy over the significance of the circadian rhythm is partially due to the conflicting associations of intervention time with clinical outcomes following major cardiac events and interventions, such as acute myocardial infarction (AMI), coronary artery bypass surgery (CABG), percutaneous coronary intervention (PCI), and aortic valve replacement (AVR). The unclear association stems from the presence of multiple biological and non-biological factors involved in the studies, such as the time lapse between the onset of cardiac symptoms to admission in hospital. Therefore, this review aims to summarize and discuss the association of circadian rhythm in major cardiac diseases and interventions.
Time of Onset of Symptoms in Myocardial Infarction and Prognosis
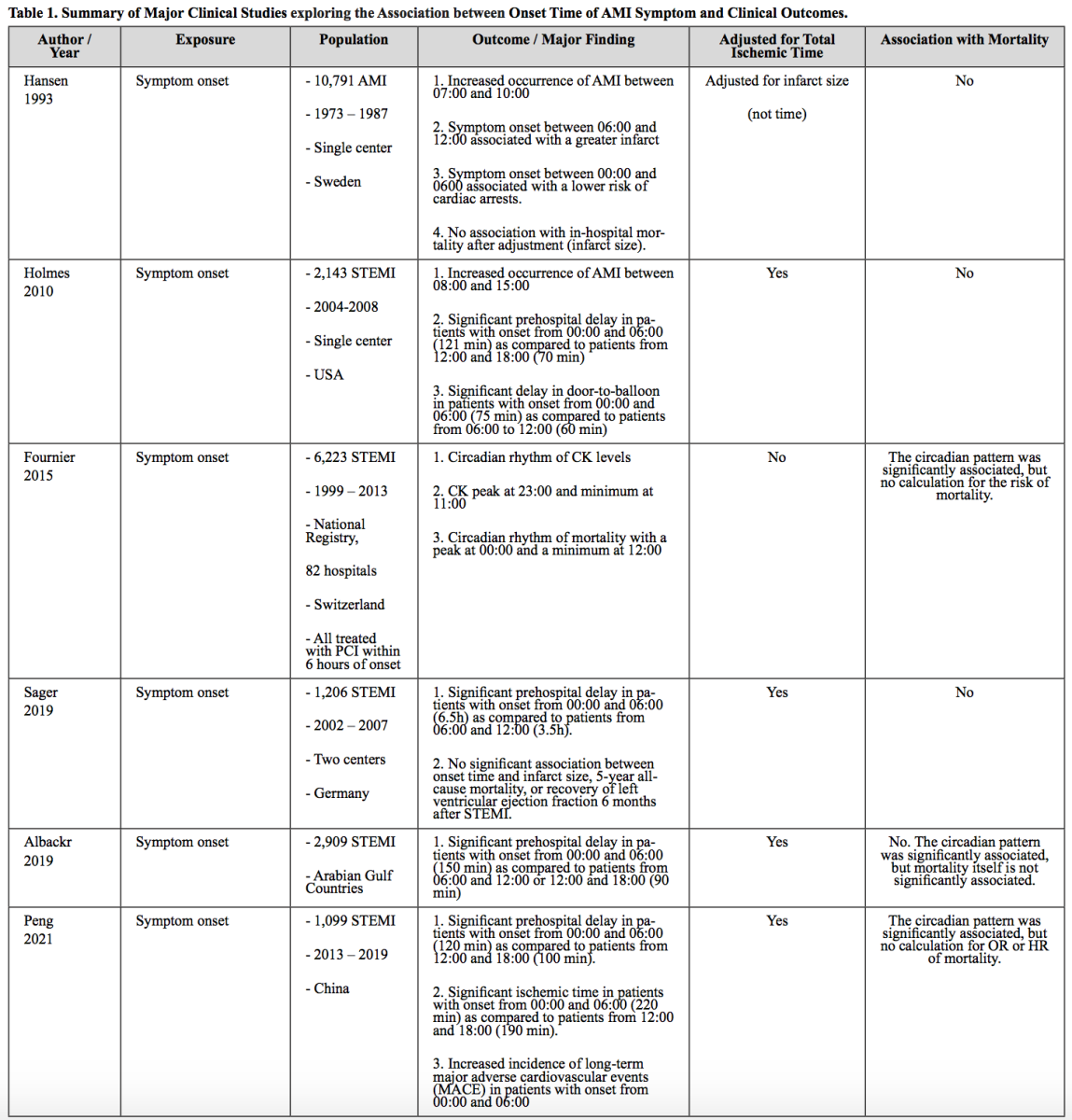
The potential importance of the circadian rhythm in cardiovascular (patho) physiology has been supported by the finding of increased AMI incidence during certain times of the day. In 1960, Master et al. (1960) described that AMI occurred with an increased frequency between 07:00 to 13:00. The increased occurrence of AMI during these times was consistently reproduced in other early studies, and it is now accepted to be an established clinical association (Pell and D'Alonzo, 1963; Muller et al., 1985; Hjalmarson et al., 1989; Willich et al., 1989). More importantly, the occurrence of AMI appears to have an oscillating pattern with a 24-hour period, also known as a circadian rhythm (Hansen et al., 1993; López Messa et al., 2004), and the mortality has been potentially associated in a circadian manner (Fournier et al., 2015). Various hypotheses have been proposed to explain the implication of the circadian rhythm in AMI and the potentially increased mortality of AMI depending on time of symptom onset. One of the plausible hypotheses is that the underlying pathological or defensive mechanisms, such as necrosis, ischemia-reperfusion injury, or cellular regeneration, are under the control of the circadian rhythm leading to a change in mortality following AMI events and subsequent reperfusion interventions (Bochaton and Ovize, 2018; Liu et al., 2021). However, another proposed explanation for the increased mortality is that non-biological factors, such as a delayed transportation to the hospital and, thus, an increased total ischemic time, can lead to worsened mortality. Indeed, patients who have AMI symptoms near midnight or early in the morning might not present to an emergency room early due to logistical issues (Khaper et al., 2018). This section of the review will focus on studies examining the possible involvement of non-biological factors on the time of onset of AMI symptoms and mortality.
A study by Hansen et al (1993) is one of the earliest papers that aimed to describe the significance of circadian rhythm on mortality following AMI. In this study, consistent with later reports, the symptoms of AMI ocurred more frequently in the morning, between 07:00 and 10:00. The time of symptom onset between 06:00 and 12:00 was associated with a greater infarct size, indicative of more severe MI. However, after adjusting for infarct size, in-hospital mortality was not associated with the time of onset of AMI symptoms. The author interpreted this result as evidence of a circadian rhythm in mortality following AMI, as infarct size is associated with oxygen demand and supply, which could be under the control of the circadian rhythm, and the statistical significance for the circadian pattern was lost after the adjustment. Although this paper was pioneering as the authors attempted to adjust the mortality with infarct size to evaluate the involvement of circadian rhythm, this interpretation is limited; the change in infarct size could be due to a significant pre-hospital delay (correlated with a prolonged total ischemic time), not just the circadian rhythm. Since there were at least two variables affecting the infarct size, total ischemic time and circadian rhythm, this study could not establish the association of time of onset of AMI symptoms with mortality. A proper statistical adjustment for total ischemic time is important to assess the impact of time of onset of AMI symptoms on mortality. In a later study performed by Holmes et al (2010), a significant pre-hospital delay in the patients with symptom onset between 00:00 and 06:00 (121 min) was observed as compared to those from 12:00 and 18:00 (70 min). After adjustment for potential confounders including the pre-hospital or total ischemic time, the mortality was not significantly associated with the onset time of AMI symptoms, suggesting that the ischemic injury, as indicated by mortality, might not have been under the influence of the circadian rhythm. Similarly, in other studies, the circadian pattern of mortality over onset time of ST-segment elevation myocardial infarction (STEMI) symptoms, was significant, but the mortality itself was not significant after adjusting for total ischemic time (Fournier et al., 2015; Albackr et al., 2019; Sager et al., 2019). Our research group previously demonstrated that the time variation over STEMI mortality is significantly observed only in the group with 120 min or a longer ischemic time and the circadian pattern was not observed in the group with a short ischemic time (Paradies et al., 2020). This result suggests that total ischemic time is a crucial component of the onset time-dependent mortality following AMI events, and thus the significance of the circadian rhythm is questionable.
In summary, it is widely accepted that there is an increased frequency of first onset of AMI symptoms in the morning. However, it is still unclear if a specific onset time of AMI symptoms is significantly associated with an altered mortality following AMI events after proper statistical adjustments, especially for total ischemic time (Table 1).
Time Variation in Percutaneous Coronary Intervention and Prognosis
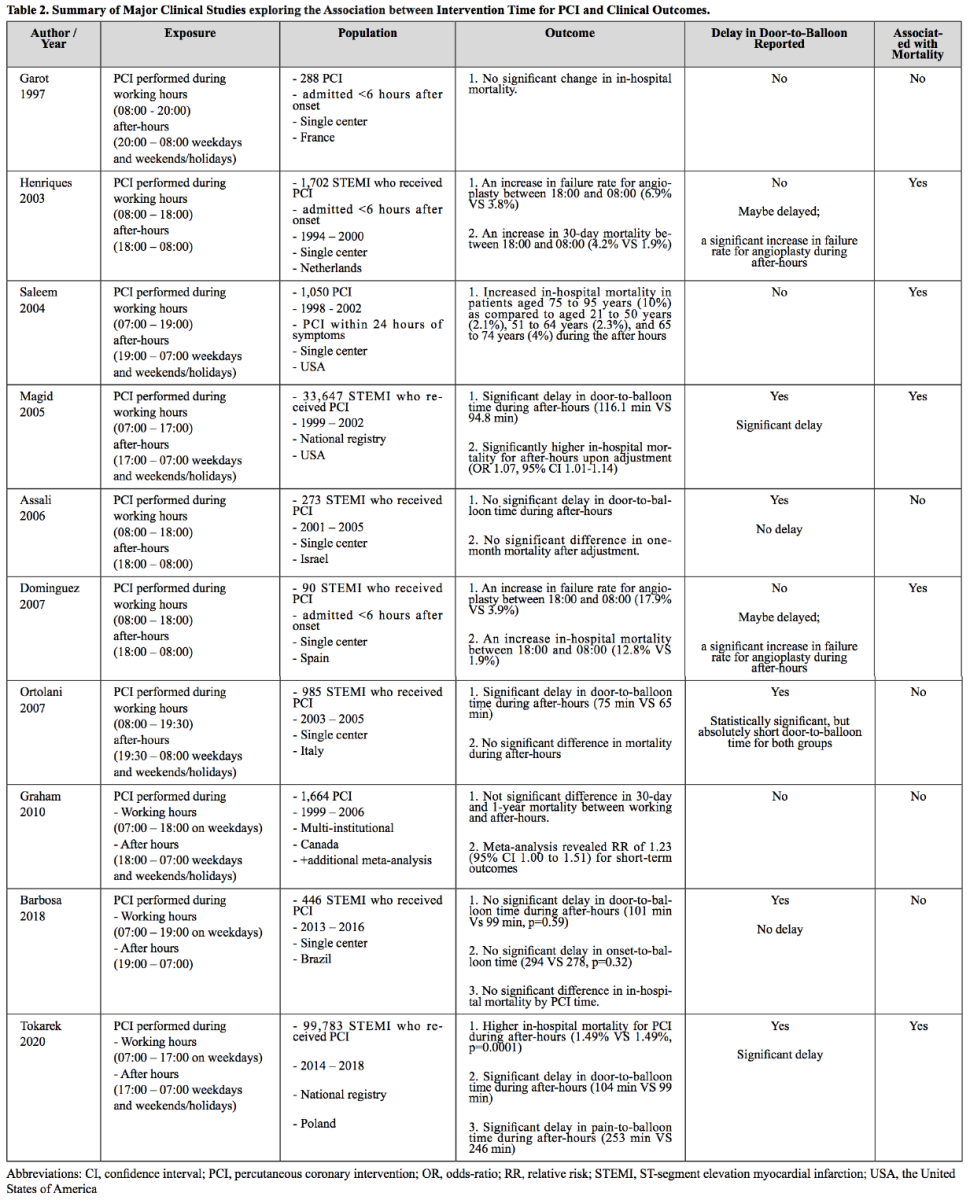
Percutaneous coronary intervention (PCI) is another method of intervention for reperfusion following AMI events. The association of PCI with clinical outcomes in AMI in relation to intervention time has been studied in the context of potential sub-optimal care for patients admitted after working-hours to examine whether patients treated after-hours are more likely to have a worse prognosis. One of the very first reports on this issue was by Garot et al. (1997) who demonstrated that there was no increased mortality in the AMI patients treated with PCI after working-hours as compared to those treated between 08:00 and 20:00. Interestingly, other studies on the same research question reported a significantly increased 30-day mortality or in-hospital mortality in patients who received PCI during after-hours. PCI performed during after-hours was associated with an increased rate of failure for angioplasty (Henriques et al., 2003; Dominguez-Rodriguez et al., 2007), a significant delay in door-to-balloon time (Magid et al., 2005), advanced age of the patients (Saleem et al., 2004), and therefore suggests that the increased mortality for the PCI performed during after-hours was presumably due to resource or patient factors. Studies reporting no difference in delay of door-to-balloon time (Assali et al., 2006; Barbosa et al., 2018; Lattuca et al., 2019) or a minimal delay (Ortolani et al., 2007) demonstrated insignificant change in mortality in the patients treated with PCI following AMI during after-hours. This result is in line with the well known importance of the short door-to-balloon time for prognosis following AMI events (Rathore et al., 2009). In summary, PCI performed during after-hours is associated with a poorer prognosis in AMI patients presumably due to an increased door-to-balloon time and increased failure rate for angioplasty. When there was no significantly delay in the door-to-balloon time, PCI performance was not associated with the intervention time, working hours, or after-hours (Table 2). Therefore, these studies collectively suggest that PCI centers are required to minimize the door-to-balloon time by optimizing the infra-structure and resources especially during the after-hours.
Time Variation in Coronary Artery Bypass Surgery and Prognosis
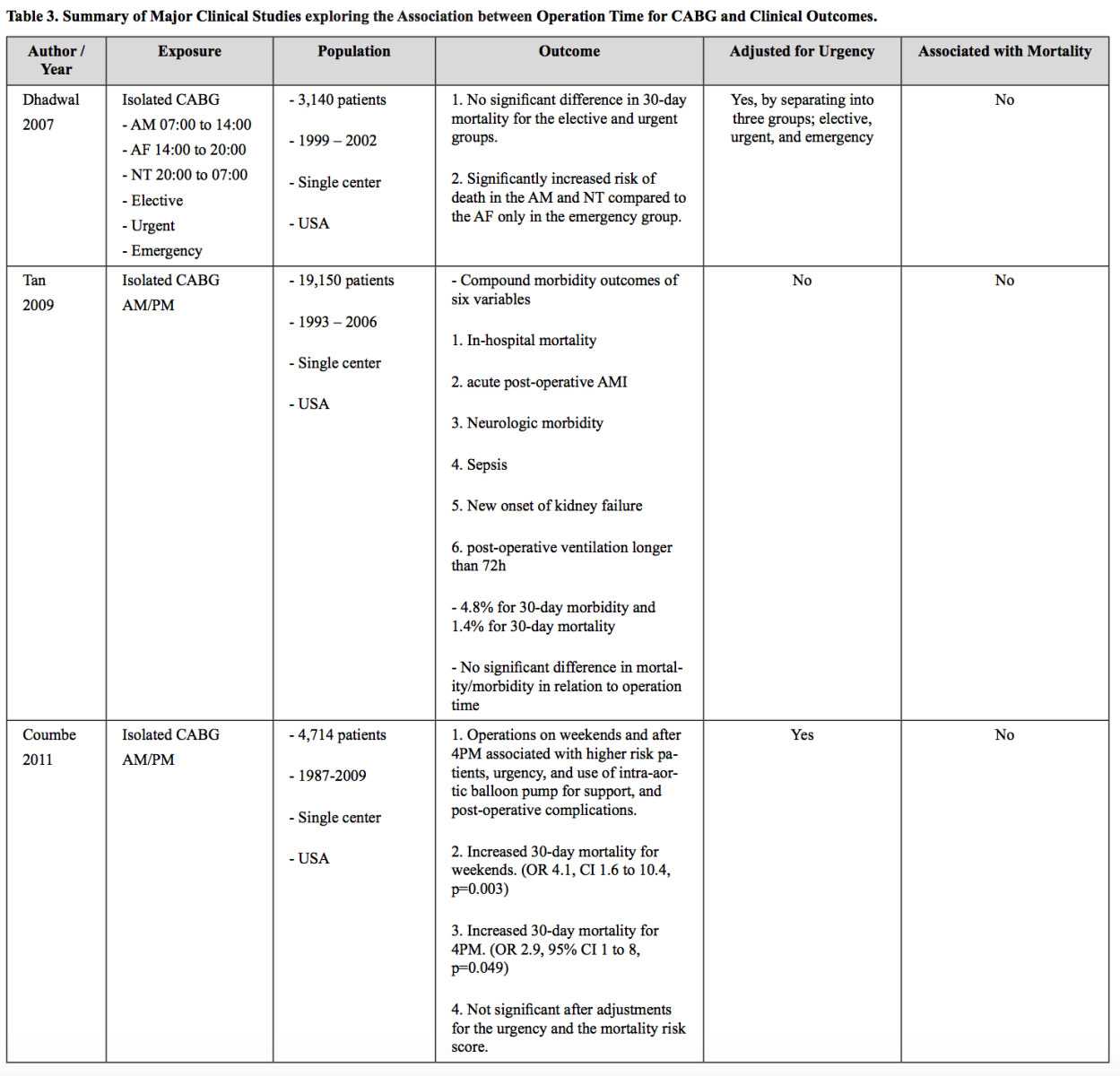
Coronary artery bypass graft surgery (CABG) is a surgical intervention for reperfusion following AMI events. Circadian rhythm has been proposed as a potential mechanism affecting the clinical outcome after CABG as the rhythm can affect ischemia-reperfusion injury and recovery. The influence of circadian rhythm over the outcome of patients undergoing cardiac surgery has been controversial. This controversy is partially due to the complexity of the data interpretation confounded by non-biological factors, such as fatigue of the surgical teams, limited resources during after-hours, and also urgent surgeries being performed at night times or weekends (Khaper et al., 2018). Dhadwal et al. (2007) resolved this issue by stratifying the CABG patients not only into times (morning, afternoon, and night) but also into urgency (elective, urgent, and emergency). There was no significant difference in 30-day mortality for the elective and urgent groups in relaionship to time. Interestingly, emergency cases performed in the morning and night were associated with an increased risk of death as compared to the afternoon group. Nonetheless, given the fact that the circadian pattern was not observed in the other two types of cases and the severity of the cases in the emergency group, surgery time did not seem to be associated with a mortality change. This result was corroborated in two other studies of over 14,000 patients consisting of both elective and urgent cases (Tan et al., 2009; Nemeth et al., 2021). Furthermore, beyond mortality outcomes, other complications, such as stroke, prolonged ventilation, renal failure, wound infection, reoperation, AMI, atrial fibrillation, and readmissions were not associated with the time of surgery. Additionally, two recent studies where the influence of the urgency of surgeries was considered for statistical adjustments (Coumbe et al., 2011) or the urgent cases were excluded (Fudulu et al., 2021), demonstrated that there was no change in mortality in relation to time of surgery. In summary, the studies collectively suggest that there is no change in mortality following CABG surgery in AMI patients in relation to time of surgery (Table 3). This result suggests that circadian rhythm may not influence clinical outcomes following CABG.
Time Variation in Aortic Valve Replacement and Prognosis
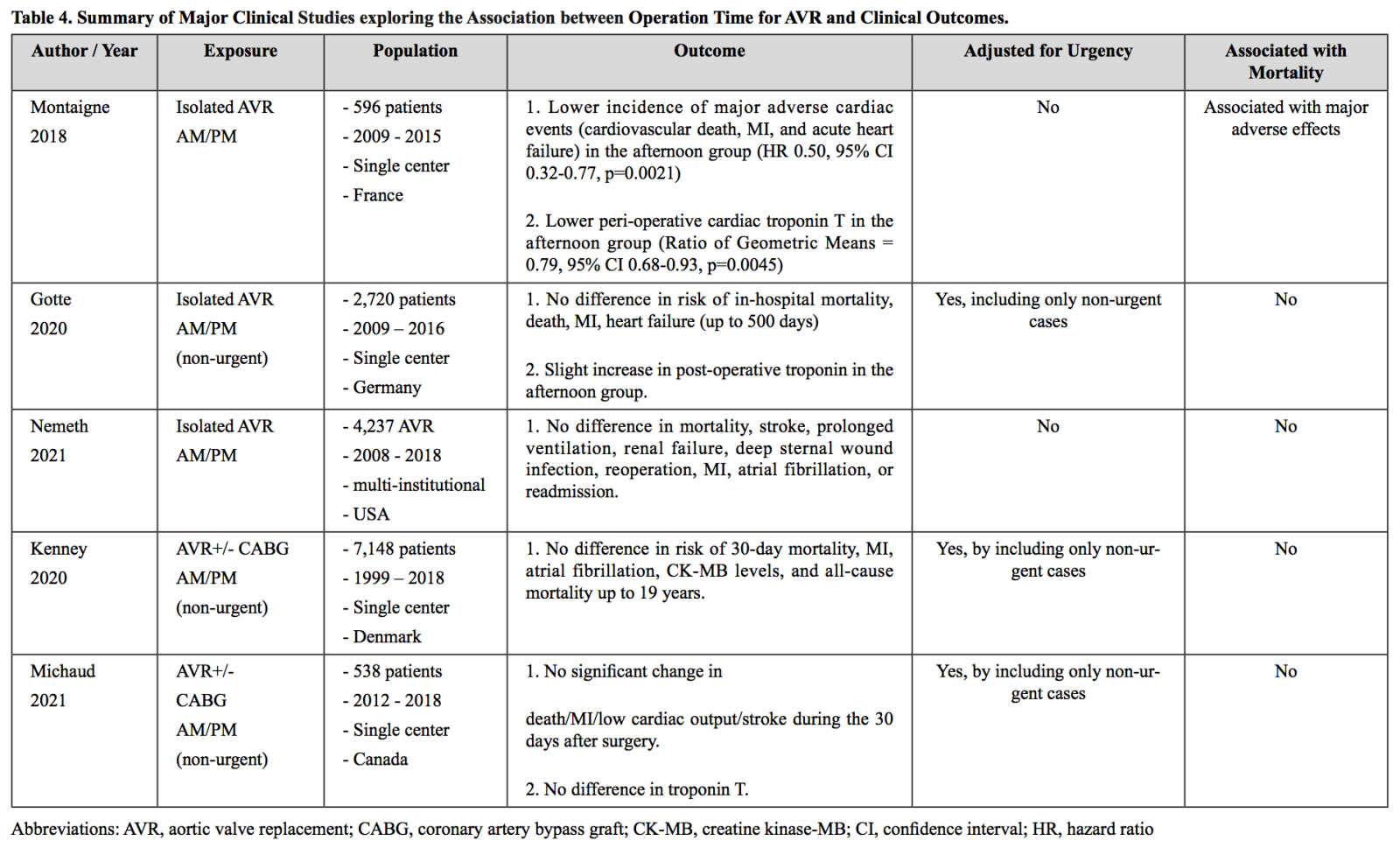
Aortic valve replacement surgery (AVR) is another cardiac surgery where circadian rhytm has been proposed to play a role. AVR requires an aortic-cross clamping, which induces ischemic injury followed by ischemia-reperfusion injury (Rossiter et al., 1974; García-de-la-Asunción et al., 2013). Due to the potential role of circadian rhythm over the reduction and oxidation balance (redox), clinical outcomes of AVR have been studied in relation to the operation time. In a single center study performed in 2018 where 596 patients received an isolated AVR, the patients who received the surgery in the afternoon had a significantly lower incidence of major cardiac adverse events, including cardiovascular death, AMI, and acute heart failure (Montaigne et al., 2018). Moreover, the afternoon group had a significantly lower peri-operative cardiac troponin T in their randomized controlled study. This result could be broadly due to the two factors: 1) circadian rhythm or 2) a so-called “human factor,” such as allocating more challenging cases in the morning group, which is commonly done in many hospitals. The authors supported their findings by performing ex-vivo experiments where the cardiomyocytes taken from the afternoon group recovered contraction better after the hypoxia-reoxygenation injury than the morning group. However, these findings and experimental data do not necessarily rule out the potential influence of other factors. Although the authors controlled for baseline factors via propensity score matching, there could be residual confounding factors that were not accounted for. Major reports after this initial one could not reproduce the significantly better outcome in the afternoon group (Götte et al., 2020; Kenney et al., 2020; Michaud et al., 2021; Nemeth et al., 2021). In a study with only non-urgent isolated AVR cases where the study design was very similar to the original study with a greater patient number, the afternoon group did not have favorable clinical outcomes, including in-hospital mortality, death, AMI, and heart failure 500 days of follow-up (Götte et al., 2020). In fact, the afternoon group had slightly elevated troponin levels, which is contrary to the previous study. Similarly, in two other studies where the non-urgent cases of AVR with or without CABG surgery were included, there was no significant change in mortality and major adverse cardiac events between the morning and afternoon groups (Kenney et al., 2020; Michaud et al., 2021). A similar finding was reported from a study that contained both urgent and non-urgent isolated AVR cases (Nemeth et al., 2021). In summary, the initial report for AVR demonstrated that patients who received AVR surgery in the afternoon had better clinical outcomes, but multiple reports after this one could not reproduce this finding even with a similar study design and a larger cohort size (Table 4). Given the common practice of performing challenging cases of cardiac surgery in the morning group, we cannot conclude that circadian rhythm may play a pivotal role at least in AVR surgery through ischemia-reperfusion injury and recovery.
Limitations and Future Directions
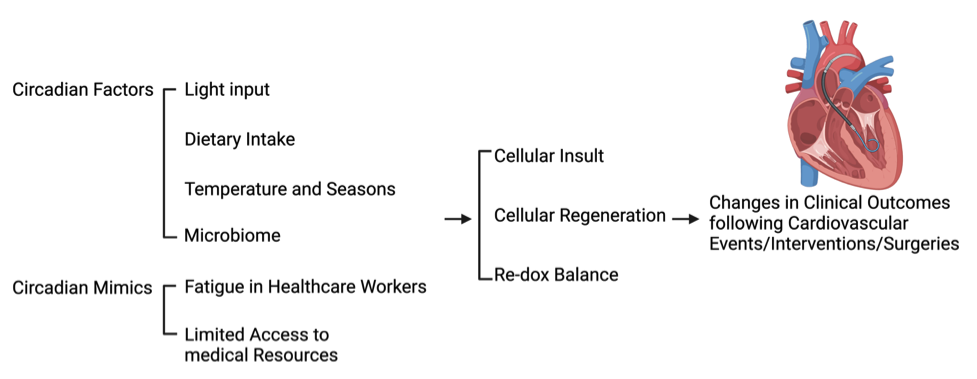
The major retrospective cohort studies mentioned in this review have been conducted in various countries in the world. As well documented in previous studies, circadian rhythm may affect physiological and pathological progression via multiple factors, including season, climate, diet, or microbiome (Nicolau et al., 1991; Dong et al., 2020; Mistry et al., 2020). Therefore, these factors should be considered for data interpretation (Figure 1).
In a new window | Download PPT
Figure 1: Potential circadian factors and mimics that affect cardiovascular clinical outcomes. Potential circadian factors and mimics, such as light input, dietary intake, temperature, microbiome, fatigue in healthcare workers, and limited access to medical resources, can affect the biological responses of the heart leading to changes in clinical outcomes following cardiac events, surgeries, and interventions.
The COVID-19 pandemic has worsened the pre-existing healthcare burdens in the world potentially negatively affecting the clinical outcomes in patients. The COVID-19 infection has been proposed to be a risk factor of cardiovascular diseases as 1) the COVID-19 may aggravate cardiovascular injuries in an acute phase or 2) patients may not seek medical help out of fear of infection, causing a significant delay in administration of urgent medical treatments (Bonow et al., 2020; Harrison et al., 2021). This tendency of avoidance for medical care could be more evident especially when there is a local surge for COVID-19 cases. Therefore, this factor needs to be considered when the clinical data spanning this period are utilized for circadian studies as the seasonal periodic pattern in clinical outcomes in this period may be affected by local COVID-19 cases, rather than biological circadian patterns.
Conclusions
While there is an extensive amount of compelling data from basic science research on the impact of circadian rhythm on cardiovascular outcomes, and with multiple clinical studies suggesting that circadian patterns may be associated with clinical outcomes for certain cardiac interventions, the overall data are still inconclusive. There are both biological factors, such as circadian rhythm, and non-biological factors, such as healthcare workers’ fatigue, limited resources, and significant delay in care confounding the picture. At present, there is still insufficient evidence that circadian rhythm affects patient outcomes in these situations. Further well-designed studies are needed to explore this relationship further.
Conflict of interests
The author(s) declare no competing interests.
Contributions
J.K. C.H.S., and D.J.H. designed the structure and contents of the review paper; J.K. and C.H.S. reviewed the literature and interpreted the data. J.K., C.H.S., and D.J.H wrote the manuscripts. D.J.H. supervised and provided critical review of the manuscript.
Acknowledgements
We thank the colleagues and staff members of Duke-NUS Medical School, SingHealth, National Heart Centre Singapore, and National University Hospital for their tremendous help on this project.
Sources of Funding
CHS was supported by the National University of Singapore Yong Loo Lin School of Medicine’s Junior Academic Faculty Scheme.
DJH was supported by the Duke-National University of Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology).
References
Junsuk Ko1
1Duke-NUS Medical School, National University of Singapore, Singapore. 2Department of Cardiology, National University Heart Centre Singapore, Singapore.
Ching-Hui Sia2,3
2Department of Cardiology, National University Heart Centre Singapore, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Derek J Hausenloy1,3,4,5,6
1Duke-NUS Medical School, National University of Singapore, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 4National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 5The Hatter Cardiovascular Institute, University College London, London, United Kingdom. 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Junsuk Ko and Ching-Hui Sia contributed equally to this article.
Corresponding author:
Professor Derek J Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg

In a new window | Download PPT
Figure 1: Potential circadian factors and mimics that affect cardiovascular clinical outcomes. Potential circadian factors and mimics, such as light input, dietary intake, temperature, microbiome, fatigue in healthcare workers, and limited access to medical resources, can affect the biological responses of the heart leading to changes in clinical outcomes following cardiac events, surgeries, and interventions.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8929 | 16 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA