Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Insights into the potential cardioprotective mechanisms of SGLT2 inhibitors
Time:2022-05-24
Number:10835
Shuo Cong1,2,3, Xavier Chan1,4, En Ping Yap1,2, Chrishan J. Ramachandra1,2, Derek J. Hausenloy1,2,3,5,6
Author Affiliations


- 1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore.
- 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.
- 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
- 4SingHealth Duke-NUS Transplant Centre, Singapore.
- 5The Hatter Cardiovascular Institute, University College London, London, UK.
- 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan.
Conditioning Medicine 2022. 5(1): 1-10.
Abstract
Heart failure (HF) is a leading cause of death and disability worldwide. As such, there is an urgent need to discover novel treatment targets that can reduce the risk of HF and improve clinical outcomes. The sodium glucose co-transporter 2 (SGLT2), which is selectively expressed in human kidney, plays a critical role in regulating glucose levels. SGLT2 is overactive in patients with type 2 diabetes mellitus (T2DM); hence, its inhibition has been proposed as a therapeutic strategy for alleviating hyperglycemia. Intriguingly, in addition to lowering glucose levels, SGLT2 inhibitors have been found to improve cardiovascular outcomes with primary effects on reducing hospitalization for HF in both T2DM and non-diabetic patients. The mechanisms underlying the cardiovascular protective effects of SGLT2 inhibitors remain unclear. The absence of SGLT2 in cardiomyocytes, albeit controversial, has prompted researchers to re-evaluate the mode of action of SGLT2 inhibitors. Several hypotheses have been proposed including the regulation of ketone body metabolism, restoration of ionic imbalances, improved mitochondrial function, and reduced inflammation, culminating in the restoration of cardiac function. In this review, we provide an overview of the clinical trials that have evaluated the use of SGLT2 inhibitors and discuss the potential mechanisms through which cardioprotection could be achieved. A deeper understanding of how SGLT2 inhibitors prevent HF could facilitate their rapid entry into routine clinical practice to improve health outcomes in HF patients.
Keywords: Heart failure, Sodium glucose co-transporter 2 inhibitors, Diabetes, Ketone bodies, Sodium-hydrogen exchanger (NHE), Oxidative stress
Abstract
Heart failure (HF) is a leading cause of death and disability worldwide. As such, there is an urgent need to discover novel treatment targets that can reduce the risk of HF and improve clinical outcomes. The sodium glucose co-transporter 2 (SGLT2), which is selectively expressed in human kidney, plays a critical role in regulating glucose levels. SGLT2 is overactive in patients with type 2 diabetes mellitus (T2DM); hence, its inhibition has been proposed as a therapeutic strategy for alleviating hyperglycemia. Intriguingly, in addition to lowering glucose levels, SGLT2 inhibitors have been found to improve cardiovascular outcomes with primary effects on reducing hospitalization for HF in both T2DM and non-diabetic patients. The mechanisms underlying the cardiovascular protective effects of SGLT2 inhibitors remain unclear. The absence of SGLT2 in cardiomyocytes, albeit controversial, has prompted researchers to re-evaluate the mode of action of SGLT2 inhibitors. Several hypotheses have been proposed including the regulation of ketone body metabolism, restoration of ionic imbalances, improved mitochondrial function, and reduced inflammation, culminating in the restoration of cardiac function. In this review, we provide an overview of the clinical trials that have evaluated the use of SGLT2 inhibitors and discuss the potential mechanisms through which cardioprotection could be achieved. A deeper understanding of how SGLT2 inhibitors prevent HF could facilitate their rapid entry into routine clinical practice to improve health outcomes in HF patients.
Keywords: Heart failure, Sodium glucose co-transporter 2 inhibitors, Diabetes, Ketone bodies, Sodium-hydrogen exchanger (NHE), Oxidative stress
Introduction
Heart failure (HF) is prevalent worldwide, with an estimated 63.4 million people suffering from the disease (Collaborators, 2018). The marked increase in comorbidities, such as hypertension, diabetes, obesity, renal dysfunction, anemia, and chronic obstructive pulmonary disease, together with high economic costs that are estimated at US$24,383 per patient, underscore an urgent need to discover novel treatment targets that can help reduce the risk of HF and improve clinical outcomes (van der Wal et al., 2017; Urbich et al., 2020).
The sodium glucose co-transporter 2 (SGLT2), which is expressed mainly at the apical membrane in proximal convoluted tubules (Balen et al., 2008), plays a critical role in regulating glucose levels by reabsorbing renal glucose (close to 90%) following filtration by the kidneys (Wright et al., 2011; Scheen, 2015). In patients with type 2 diabetes mellitus (T2DM), filtration of glucose by the kidneys and tubular glucose reabsorption is augmented, which in turn can lead to sustained hyperglycemia (Vallon and Thomson, 2017). Hence, inhibition of SGLT2 was first proposed as an intervention strategy to reduce blood glucose levels in these patients by promoting glucosuria (Tat and Forest, 2018). Remarkably, in addition to lowering glucose, SGLT2 inhibition has been reported to improve cardiovascular outcomes with primary effects on HF hospitalization in both T2DM and non-diabetic patients (Lytvyn et al., 2017; Zannad et al., 2020; Ferrannini et al., 2021), suggesting that this class of drugs confer cardiovascular protective effects (summarized in Table 1), although the mechanisms underlying these effects remain unclear.
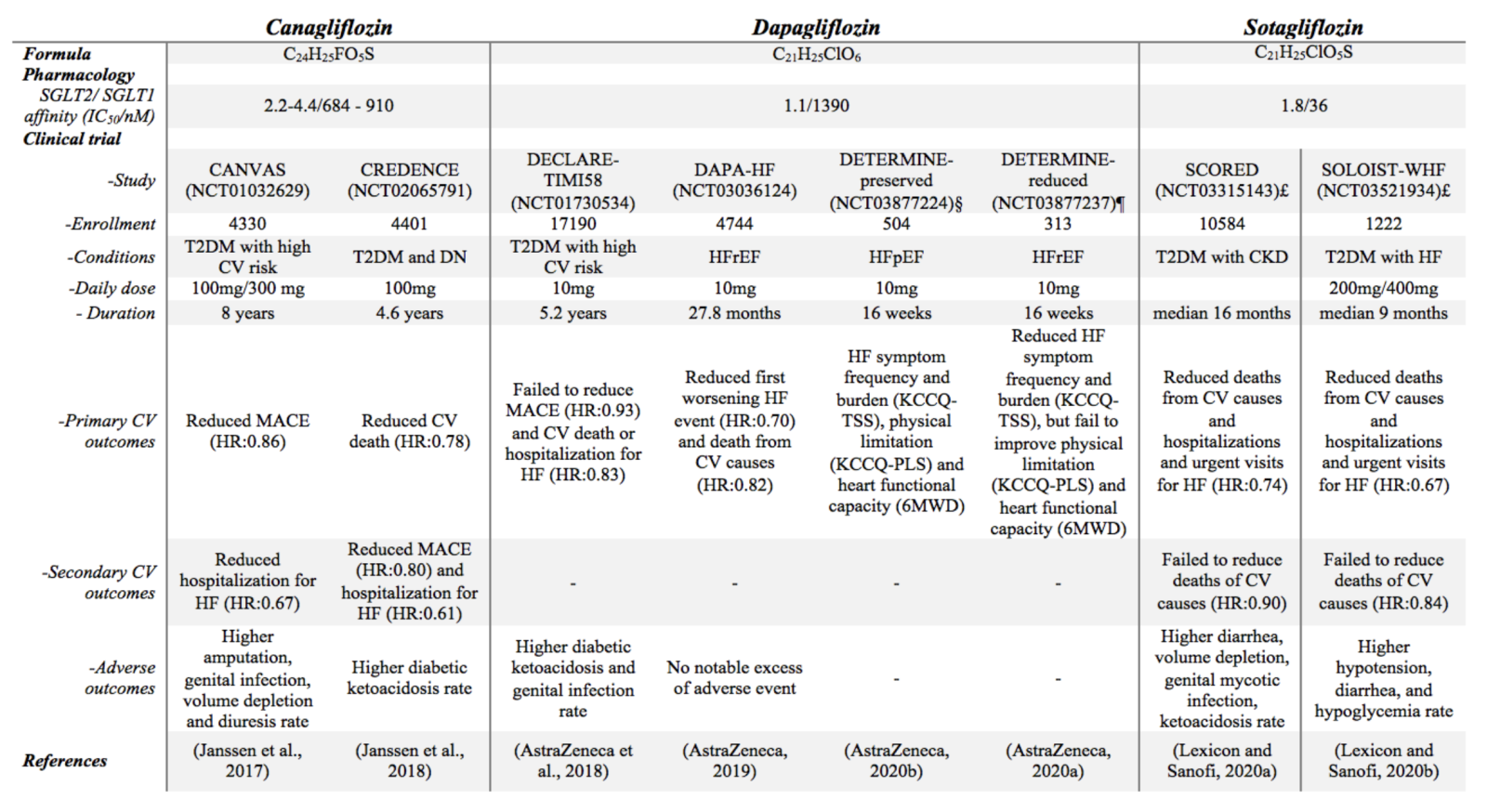
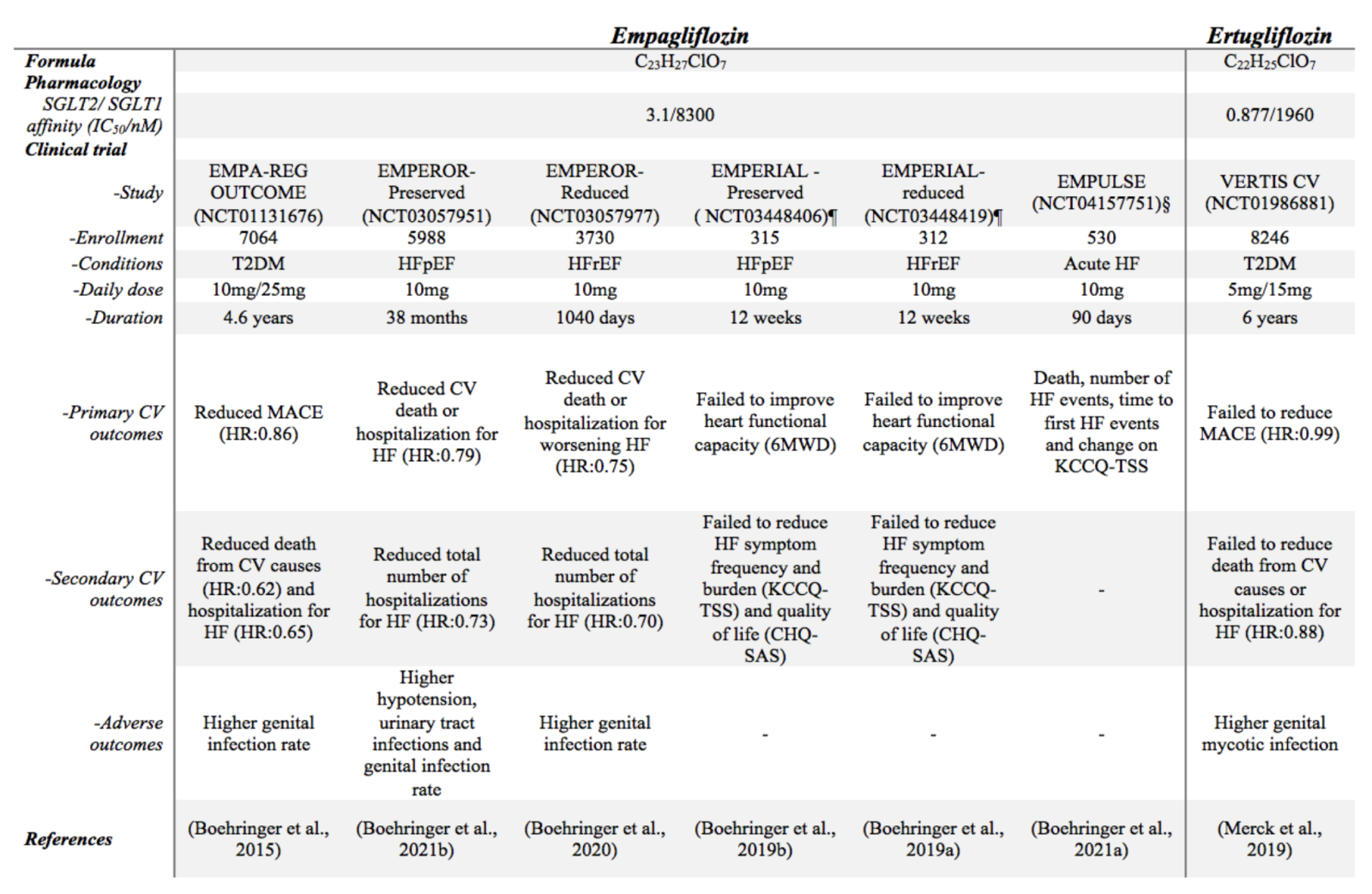
Table 1: Summary of randomized double-blinded multi-centre large size clinical trials focusing on cardiovascular benefits of SGLT2 inhibitors
£ Terminated trial but outcome reported
§ Completed trial but no data disclosed
¶ Completed trial, data disclosed but no official report
Abbreviations: 6MWD, 6-minute walk distance; CHQ-SAS, chronic heart failure questionnaire self- administered standardized format; CKD, chronic kidney disease; CV, cardiovascular; DN, diabetic nephropathy; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; MACE, major adverse cardiovascular event; KCCQ-12, Kansas City Cardiomyopathy Questionnaire–12 item; KCCQ-PLS, Kansas-City cardiomyopathy questionnaire-physical limitation score; KCCQ-TSS, Kansas-City
In this review article, we summarize the clinical applications of SGLT2 inhibitors and discuss potential mechanisms through which they may function, with special emphasis on effects on cardiac metabolism, ion channel function, mitochondrial function, and inflammation (Figure 1). We also discuss current controversies surrounding SGLT2 inhibitors, with the aim of gaining deeper insight into their mode of action, which may facilitate their rapid entry into clinical practice to be used as a standardized therapeutic modality for HF patients.
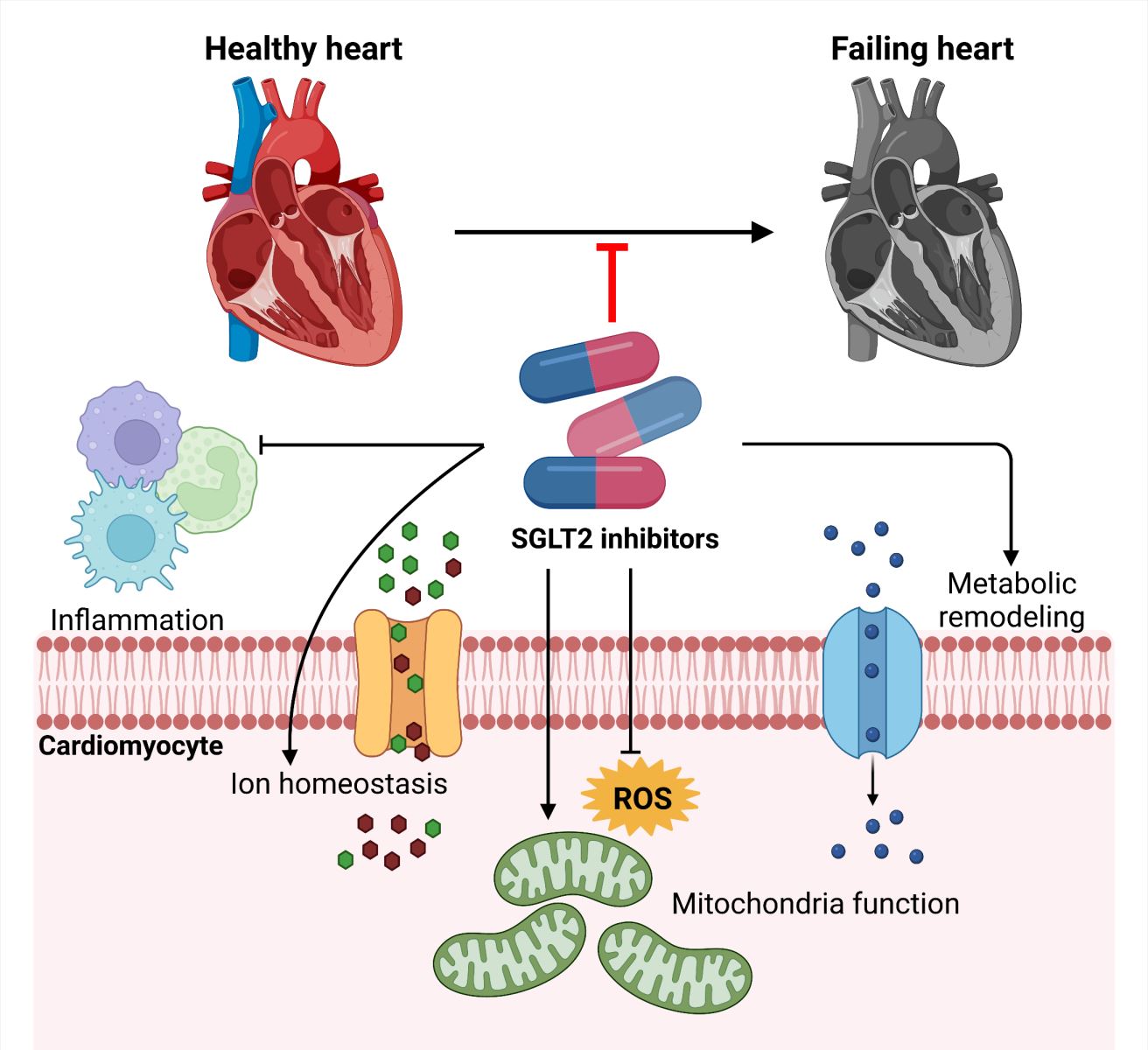
In a new window | Download PPT
Figure 1: Summary of potential mechanistic actions of SGLT2 inhibitors.
An overview of clinical trials of SGLT2 inhibitors
SGLT2 inhibitors mediate beneficial pleiotropic effects in T2DM patients
Phlorizin, found in the bark of apple trees, was the first natural SGLT inhibitor discovered about a century ago (Ehrenkranz et al., 2005). Consistent with its glucose lowering ability, phlorizin was shown to reduce hyperglycemia and restore insulin sensitivity in partially pancreatectomized diabetic rats, with no alteration in insulin levels (Rossetti et al., 1987). However, when studied in humans, phlorizin was considered to be a suboptimal drug candidate, limited by scarce bioavailability following oral administration and the development of significant side effects in the gastrointestinal tract (Choi, 2016). Continuing efforts in the field have superseded phlorizin and led to the discovery of a myriad of selective SGLT2 inhibitors such as dapagliflozin, canagliflozin, and empagliflozin (Plodkowski et al., 2015), as well as SGLT1/2 dual inhibitors like sotagliflozin (Cefalo et al., 2019). Notably, dapagliflozin, which acts as a competitive inhibitor of SGLT2 by impeding renal glucose reabsorption was the first licensed drug to be considered as an alternative for patients not responsive to metformin, and its therapeutic efficacy has been validated in large randomized double-blinded cohort studies (Saeed and Narendran, 2014; Fioretto et al., 2015).
Based on its unique mechanism of enhancing glucose excretion, SGLT2 inhibitors have been recognized as a treatment modality to improve glucose control, with a similar risk of hypoglycemia when compared to metformin and dipeptidyl peptidase-4 inhibitors (Hsia et al., 2017). However, it was later discovered that in addition to exerting direct effects on glycemic control, SGLT2 inhibitors may also play an active role in lowering blood pressure, increasing hemoglobin concentration, and reducing body weight (Thiele et al., 2021). Moreover, researchers have uncovered a range of beneficial effects exerted by SGLT2 inhibitors, including reductions in volume load and inflammation, as well as increased renal protection that is essential for preventing the development and progression of atherosclerosis, a major complication of diabetes (Kang et al., 2020). These findings led to the initiation of several studies that evaluated SGLT2 inhibitors (such as canagliflozin, dapagliflozin or empagliflozin) in T2DM patients. These studies have consistently reported that these classes of drugs can reduce the risk of major adverse cardiovascular events (MACE) including hospitalization for HF (D'Andrea et al., 2020), as well as attenuate the progression of chronic kidney disease (Khunti, 2021).
The EMPA-REG OUTCOME clinical trial in 2015 investigated whether 10 mg or 25 mg of empagliflozin daily (in addition to standard care) for an average of 3 years would have beneficial effects in T2DM patients with high cardiovascular risk. Crucially, empagliflozin was found to lower the incidence rate of primary outcome events, including death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke. Moreover, empagliflozin was found to significantly the reduce risk of HF hospitalization in T2DM patients when compared to the placebo group (Zinman et al., 2015). Similar findings were reported in another multicenter randomized double-blinded clinical trial (CANVAS program) that evaluated the therapeutic efficacy of canagliflozin in T2DM patients with high cardiovascular risk, whereby a reduction in the risk of MACE (a composite of cardiovascular death or hospitalization for worsening heart failure) was observed, albeit with non-significant reductions in adverse renal outcomes (Neal et al., 2017). Moreover, the SOLOIST-WHF study, which evaluated the effects of SGLT1/2 dual inhibitor sotagliflozin revealed superior outcomes as evidenced by drastic reductions (>30%) in cardiovascular-related death and HF-triggered hospitalization in T2DM patients with history of HF (Bhatt et al., 2021). Finally, a recent study comparing the therapeutic advantages between metformin and SGLT2 inhibitors revealed a lower rate of short/mid-term cardiovascular events for patients prescribed the latter (Fralick et al., 2021). Collectively, these studies demonstrate profound benefits exerted by SGLT2 inhibitors in T2DM patients; however, their potential side effects such as a higher rate of genital infection and diabetic ketoacidosis (Musso et al., 2020) warrants further mechanistic understanding on the mode of action of these classes of drugs.
SGLT2 inhibitors mediate cardiovascular protection in the absence of diabetes
Following the initial success of SGLT2 inhibitors in T2DM patients, researchers have also evaluated their therapeutic efficacy in non-diabetic patients. Remarkably, SGLT2 inhibitors were found to improve HF outcomes and reduce chronic renal failure in patients without T2DM (Kang and Jardine, 2021), which has led to the notion that SGLT2 inhibitors could potentially be used to treat conditions beyond diabetes. As such, additional clinical trials were initiated to evaluate the therapeutic efficacy of SGLT2 inhibitors in patients with established HF, regardless of the presence or absence of T2DM. In the DAPA-HF trial in 2017 (McMurray et al., 2019a), treatment of HF patients (~18 months) with reduced ejection fraction, lowered the risk of worsening HF and death from cardiovascular causes by about 30%. Moreover, there were no differences in the cardiovascular protective effects exerted by dapagliflozin treatment, irrespective of diabetic status (McMurray et al., 2019b). The EMPEROR-Reduced trial was also initiated in the same time period and evaluated the use of empagliflozin in similar patient cohorts, irrespective of their diabetic profile (Packer et al., 2020). Findings from this study were comparable to those observed in the DAPA-HF trial; however, empagliflozin was also associated with additional benefits, such as reduced risk of MACE and decline in renal function (Packer et al., 2020).
Heart failure with preserved ejection fraction (HFpEF) is poised to be the leading form of HF; and yet, there is no available therapy that can convincingly improve patient outcomes (McDonagh et al., 2021). Therefore, evaluation of SGLT2 inhibitors in this patient subgroup is an attractive concept. Although the DELIVER trial, which is investigating the effects of dapagliflozin is yet to be concluded (Solomon et al., 2021), the EMPEROR-Preserve is the first clinical trial to demonstrate significant beneficial effects in HFpEF patients (Anker et al., 2020), where Empagliflozin was found to reduce the risk of MACE (21% lower relative risk) that corelated with reduced risk of HF hospitalization in ~6000 patients (after a median 26.2-month follow-up) with class II-IV HF and an ejection fraction of more than 40% (Anker et al., 2021). Collectively, these findings together with the outcomes from other concluded clinical trials, reinforce the possibility that SGLT2 inhibitors do not discriminate between the presence or absence of diabetes. However, the mode of action of these classes of drugs in preventing the progression of HF remain largely unknown, and warrants further investigation, especially if SGLT2 inhibitors are to be used as a treatment modality for non-diabetic patients (Joshi et al., 2021).
Potential mechanisms underlying the effects of SGLT2 inhibition
SGLT2 inhibition regulates cardiometabolic remodeling
Under normal physiological conditions, long-chain fatty acids are the dominant substrates utilized by the heart for energy production; however, metabolic flexibility also allows for the utilization of glucose, lactate, ketone bodies, and amino acids in response to biological and physiological demands (Karwi et al., 2018). Conversely, cardiometabolic flexibility is impaired in HF patients, and apart from alterations in glucose and fatty acids uptake/oxidation (Davila-Roman et al., 2002; Tuunanen et al., 2006; Neglia et al., 2007), changes in ketone body and branched chain amino acid (BCAA) utilization (marginal substrates in healthy conditions) have been observed in pre-clinical models of HF (Aubert et al., 2016). Consistently, an increase in ketone body oxidation (Bedi et al., 2016), with reduced BCAA catabolism (Lopaschuk and Ussher, 2016) has been reported in failing human hearts. Hence, it could be postulated that SGLT2 inhibition may lead to rebalancing of fuel selection and restoration of substrate utilization efficiency, and this may account for the salvage effect observed in the failing heart (Ferrannini et al., 2016; Mudaliar et al., 2016; Wu et al., 2019).
In a small randomized, double-blind, placebo-controlled crossover study, SGLT2 inhibition was found to direct myocardial substrate utilization away from glucose towards other sources, but it did not affect myocardial free fatty acids uptake in T2DM patients (Lauritsen et al., 2021). This was in contrast to findings from a large-scale proteomics screen of plasma samples from participants with differing tolerance to glucose that showed SGLT2 inhibitors affecting the levels of fatty acid-binding protein, leptin receptor, and insulin-like binding factor protein, implying changes in utilization of fatty acids (Ferrannini et al., 2020). Moreover, analysis of plasma metabolites from T1DM patients revealed empagliflozin to increase tricarboxylic acid (TCA) cycle metabolites and biosynthesis of unsaturated fatty acids, supporting a shift in metabolic substrate utilization and improving mitochondrial function (Liu et al., 2021). In another prospective study, untargeted metabolomics before and after empagliflozin treatment (1 month) revealed an activation of the TCA cycle, increased degradation of BCAAs and ketogenic amino acids, as well as elevated β-hydroxy-butyrylcarnitine levels in T2DM patients with cardiovascular disease (Kappel et al., 2017). While these findings imply an elevation of ketone bodies and BCAA catabolism to be the basis of increased turnover rate of the TCA cycle, the metabolic profile mediated by SGLT2 inhibition could be compared to those observed during aestivation and caloric restriction (Marton et al., 2021; Op den Kamp et al., 2021).
To understand the potential significance of endogenous ketosis that is associated with SGLT2 inhibition (Selvaraj et al., 2020), several studies have been conducted, albeit with contrasting findings. For instance, in a non-diabetic fasted HF pig model, empagliflozin was found to switch myocardial fuel utilization away from glucose towards ketone bodies, fatty acids, and BCAA, which was associated with improved myocardial energetics, enhanced left ventricular (LV) systolic function, and attenuated adverse LV remodeling (Santos-Gallego et al., 2019). Conversely, in a diabetic mouse model, no changes in cardiac ketone bodies and serum BCAA metabolites were detected (Moellmann et al., 2020) following empagliflozin treatment, nor were the cardiac ketone oxidation rates affected (Verma et al., 2018). Similarly, in diabetic, obese rats with spontaneously hypertensive HF, empagliflozin treatment led to a reduction in afterload (with no alterations in myocardial function), and was unexpectedly associated with reduced myocardial ketone body utilization, despite elevated fasting blood β-hydroxybutyrate levels (Abdurrachim et al., 2019). While these discrepancies could be attributed to the presence and duration of fasting, the type of model used, and the stage of the disease, future studies are required to elucidate the roles of ketones in preserving cardiac function in response to SGLT2 inhibition.
SGLT2 inhibition regulates ion homeostasis
Dysregulated cardiomyocyte ion homeostasis, especially elevated cytosolic [Na+] levels and abnormal [Ca2+] handling, result in impaired cardiac excitation-contraction coupling, and crucially, mediate the development of cardiac hypertrophy and HF (Pogwizd et al., 2003; Schwinger et al., 2003; Kohlhaas et al., 2010; Marks, 2013). Hence, it has been postulated that SGLT2 inhibitors may improve cardiac function by normalizing ionic imbalance through off-target effects on ion channels. In support of this, empagliflozin was found to reduce myocardial cytosolic [Na+] and [Ca2+] levels, and enhance mitochondrial [Ca2+] concentrations by directly inhibiting the cardiac Na+/H+ exchanger (NHE) in isolated ventricular myocytes (Baartscheer et al., 2017). These findings have been supported by other studies, whereby empagliflozin was found to exert cardioprotection during ischemia in ex vivo mouse hearts by inhibiting NHE, an effect that was lost in the presence of an NHE1 inhibitor (Uthman et al., 2019). Moreover, acute exposure to empagliflozin resulted in decreased NHE activity in human atrial cardiomyocytes of HF patients with elevated NHE1 levels (Trum et al., 2020). Although these studies lend support that SGLT2 inhibitors mediate cardioprotection via inhibition of myocardial NHE1 or intracellular [Na+], contrasting findings have also been reported. For instance, empagliflozin (over a wide range of concentrations) failed to inhibit cardiac NHE1 activity or alter intracellular [Na+] levels in isolated rat ventricular cardiomyocytes (Chung et al., 2020). Interestingly, the late component of the cardiac sodium channel current (late-INa) has also been implicated as a target of SGLT2 inhibitors (Philippaert et al., 2021), and whether these classes of drugs also regulate other Na+ channels remains to be determined.
Apart from regulating [Na+], SGLT2 inhibitors have also been found to mitigate abnormal [Ca2+] handling that is observed in HF. Ca2+/calmodulin dependent kinase II (CaMKII) activation, brought about by rises in cytosolic [Ca2+] is considered a hallmark of the failing heart, resulting in [Ca2+] leak from the sarcoplasmic reticulum, which impairs cardiac excitation-contraction coupling, and eventually leads to HF (Ai et al., 2005). Interestingly, 24-hour empagliflozin treatment was found to reduce CaMKII activity and attenuate phosphorylation of the ryanodine receptor in mouse HF myocytes (Mustroph et al., 2018). In other studies, empagliflozin was reported to alleviate LV diastolic function and reduce mortality in mice with diabetic cardiomyopathy (Moellmann et al., 2020). Moreover, the beneficial effects observed in this study were attributed to suppression of CaMKII activation, with subsequent reductions in spontaneous [Ca2+] leakage. Finally, inhibition of SGLT2 in aged cardiomyocytes (where it was found to be markedly increased), led to restoration of [Ca2+] homeostasis and loading of [Ca2+] in the mitochondria (Olgar et al., 2020).
Conversely, in human HF cardiomyocytes, improvements in diastolic function following empagliflozin treatment was found to be independent of changes in [Ca2+] transient amplitude and diastolic [Ca2+] levels, but was attributed to reduced myofilament passive stiffness (Pabel et al., 2018). Similarly, empagliflozin had no effects on [Ca2+] transient amplitudes, sarcoplasmic [Ca2+] load, and diastolic sarcoplasmic [Ca2+] leak in healthy cardiomyocytes derived from human induced pluripotent stem cells (iPSCs) (Pabel et al., 2020). On the contrary, empagliflozin was found to reduce [Ca2+] transient amplitude and improve [Ca2+] re-uptake in iPSC-derived cardiomyocytes cultured in a hyperglycemic environment (Ng et al., 2018), implying that modulation of cardiomyocyte [Ca2+] by SGLT2 inhibitors may be dependent on the type of cellular insult, which necessitates the need for further investigation.
SGLT2 inhibition regulates mitochondrial function
Apart from regulating cardiometabolism and ion homeostasis, it has been postulated that SGLT2 inhibitors may exert cardioprotection by improving mitochondrial function, increasing mitochondrial numbers, and by mediating protection against oxidative stress. In support of this, empagliflozin was found to reduce mitochondrial DNA (mtDNA) damage and enhance mitochondrial biogenesis by restoring PGC1-α expression, which in turn was associated with stabilization of myocardial glucose and fatty acids uptake/oxidation (Yurista et al., 2019). Moreover, SGLT2 inhibition was found to restore the size and quantity of mitochondria in diabetic rat hearts via alleviation of reactive oxygen species (ROS) (through upregulation of SOD2 and catalase) and restoration of autophagy by suppressing mitochondrial fission (Mizuno et al., 2018). In other studies, dapagliflozin has been reported to normalize mitochondrial membrane potential and expression of fusion–fission proteins (mitofusion-1, mitofusion-2, and fission-1) and to reduce production of ROS and reactive nitrogen species (RNS), thereby improving cardiac function in the setting of metabolic syndrome, diabetes, and obesity (Durak et al., 2018). Similarly, treatment with dapagliflozin before an ischemic event attenuated mitochondrial ROS production and swelling caused by ischemia-reperfusion injury, as well as mediated an increase in the mitochondrial fusion protein, OPA1, and carnitine palmitoyltransferase 1, a mitochondrial protein associated with metabolism (Lahnwong et al., 2020).
Treatment of diabetic mice with empagliflozin has also been found to reduce infarct size and interstitial fibrosis, as well as prevent cardiomyocyte apoptosis that resulted in improved cardiac microvascular protection (Zhou et al., 2018). In this study, the beneficial effects exerted by SGLT2 inhibition were attributed to restoration of mitochondrial membrane potential and attenuation of oxidative stress via activation of the 5’ adenosine monophosphate-activated protein kinase (AMPK) pathway. AMPK activation has been shown in other studies to exhibit cardioprotection by inhibiting mitochondrial fission (Zhou et al., 2018), reducing mitochondrial superoxide production (Lu et al., 2020), and by inducing mitochondrial biogenesis (Guo et al., 2020). Moreover, in an obese rat model of metabolic syndrome and exercise-induced pulmonary hypertension, increases in mitochondrial ROS and RNA degradation of nuclear factor Y α subunit, NFY (that was dependent on miR-193b), and reduction in soluble guanylate cyclase β1 subunit (sGCβ1) levels were shown to be reversed following empagliflozin treatment (Satoh et al., 2021). Recently, treatment of mitochondria from mice fed on high-fat, high-sucrose diet with ertugliflozin was shown to preserve ATP production and suppress the release of mitochondrial ROS, as evidenced by diminished levels of myocardial 4‐hydroxynonenal (an oxidative stress and lipid peroxidation marker) (Croteau et al., 2021). Finally, in a doxorubicin-induced cardiotoxicity mouse model, SGLT2 inhibitors were reported to activate the Beclin1-TLR9-SIRT3 axis, where mitochondrial SIRT3 was increased and TLR9 activation was elevated to facilitate interactions with Beclin1 and protect against apoptosis and ROS (Wang et al., 2020). Collectively, these findings support potential mitoprotective properties associated with SGLT2 inhibitors where restoration of mitochondrial function may help to preserve myocardial energetics and redox status in the failing heart.
SGLT2 inhibition regulates inflammation
SGLT2 inhibitors have also been associated with anti-inflammatory properties. In support of this, empagliflozin was shown to augment anti-inflammatory M2 marker protein expression in macrophages and reduce expression of tumor necrosis factor-α and inducible nitric oxide synthase (iNOS) in both in vitro and in vivo mouse models following stimulation with lipopolysaccharide (Koyani et al., 2020). Consistent with antioxidant and anti-inflammatory properties, empagliflozin was also found to limit myocardial infarction through a signal transducer and activator of transcription 3 (STAT3)-dependent mechanism, with concurrent reductions in interleukin (IL)-6 and iNOS (Andreadou et al., 2017). Similarly, dapagliflozin has demonstrated cardioprotection by elevating STAT3 activity, increasing anti-inflammatory myocardial IL-10 levels, and by polarizing macrophages toward an anti-inflammatory M2 phenotype (Lee et al., 2017). Finally, dapagliflozin has demonstrated anti-inflammatory and anti-fibrotic properties by reducing inflammasome activation and fibrosis respectively, which in turn led to the preservation of LVEF in a T2DM mouse model (Ye et al., 2017). In summary, while these findings associate an anti-inflammatory function of SGLT2 inhibitors, these beneficial effects are likely to be secondary with regard to global restoration of cardiac function.
Controversies and future directions
Numerous pre-clinical and clinical studies have consistently reported improved HF outcomes (irrespective of diabetes) following SGLT2 inhibitor therapy. Additionally, SGLT2 inhibition (acute and chronic) in the setting of ischemia-reperfusion injury, has also been associated with reduced infarct size and improved cardiac function, attributable to lowered [Na+] and [Ca2+], NHE inhibition, STAT3 and AMPK activation, CamKII inhibition, reduced inflammation and oxidative stress (Andreadou et al., 2020). Interestingly, certain SGLT2 inhibitors have been associated with better clinical outcomes than others. In support, a meta-analysis of 10 cardiovascular and renal outcomes revealed empagliflozin, canagliflozin, and dapagliflozin to be strongly associated with a reduced incidence of all-cause and cardiovascular-related mortality, whereas ertugliflozin or sotagliflozin did not exert such effects (Odutayo et al., 2021). Though the differential effects of these inhibitors could be due to their affinity for the SGLT2 transporter, another meta-analysis unexpectedly revealed decreasing benefit on HF events with increasing SGLT2 selectivity, albeit the latter having no effect on mortality (Tager et al., 2021). Moreover, little evidence supports the baseline risk of patients that influence the treatment effects for all-cause and cardiovascular-related mortality mediated by classes of drugs (Odutayo et al., 2021). As such, it is still unclear why certain SGLT2 inhibitors are more efficient at preventing mortality than others.
Importantly, cardiomyocytes do not express SGLT2 (Abdul-Ghani et al., 2016), and hence, direct improvements on cardiomyocyte energetics and contractility are debatable, with such phenomena likely arising from off-target effects. The expression of SGLT1 in human and rodent cardiomyocytes, and its up-regulation in several pathological conditions such as T2DM, hypertrophy, and HF, further confound matters, allowing for the postulation that SGLT2 inhibitors may mediate cardioprotection via indirect targeting of SGLT1 (Kaplan et al., 2018). Moreover, though current findings support a mitoprotective role of SGLT2 inhibitors, it remains to be determined whether this is due to direct regulation of mitochondrial dynamics and function, or secondary in response to global improvements in cardiac function. The effects of SGLT2 inhibitors on mitochondrial function could be readily tested in human iPSC-derived cardiomyocytes, which have been shown to demonstrate changes in mitochondrial morphology and metabolic flexibility (Ramachandra et al., 2018). Moreover, these human cellular models have been successful in identifying novel mechanisms and treatment targets in a patient-specific manner (Mehta et al., 2014; Mehta et al., 2018; Ramachandra et al., 2019; Cong et al., 2020; Ramachandra et al., 2020; Ramachandra et al., 2021), supporting their potential for unravelling the mode of action of SGLT2 inhibitors. In summary, while the aforementioned studies support a myriad of potential beneficial off-target effects, the possibility of adverse side effects mediated by these classes of drugs cannot be excluded, as SGLT2 inhibitor usage though capable of lowering all-cause and cardiovascular-related mortality has also been associated with increased levels of hematocrit and creatinine (Chambergo-Michilot et al., 2021).
Conclusion
Given their repeated success at reducing rates of HF hospitalization and cardiovascular-related mortality, SGLT2 inhibitors have tremendous potential to transform patient care. While these classes of drugs have been proposed to (i) restore metabolic disturbances, (ii) normalize ionic imbalances, (iii) improve mitochondrial function, and (iv) reduce inflammation, further studies are needed to determine as to how and where (e.g., cardiomyocytes, endothelial cells, inflammatory cells) these phenomena occur. Such investigations could be performed in various cellular models subjected to different forms of insults (e.g., high-glucose, hypoxia). Finally, though it is clearly evident that SGLT2 is a multifaceted target for cardioprotection, only by elucidating the mode of action of SGLT2 inhibitors can healthcare professionals circumvent adverse side effects and facilitate their rapid entry into clinical practice to be used as a standardized therapeutic modality for improving outcomes of HF patients.
Conflict of interest
The authors declare they have no conflict of interests.
Acknowledgements
Derek Hausenloy is supported by Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). Chrishan Ramachandra is supported by the SingHealth Duke-NUS Academic Medicine Research Grant (AM/TP033/2020 [SRDUKAMR2033]) and the Khoo Bridge Funding Award (Duke-NUS-KBrFA/2022/0059).NUS Academic Medicine Research Grant (AM/TP033/2020 [SRDUKAMR2033]).
Dr. Derek J. Hausenloy and Chrishan J. Ramachandra, who serve as an editor for Condtionimg Medicine, did not particpate at any level in the editorial review of this manuscript.
Handling editor of this article: Cesario V. Borlongan
References
Shuo Cong1-3
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Xavier Chan1,4
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 4SingHealth Duke-NUS Transplant Centre, Singapore.
En Ping Yap1,2
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.
Chrishan J. Ramachandra1,2
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore.
Derek J. Hausenloy1-3,5,6
1National Heart Research Institute Singapore, National Heart Centre Singapore, Singapore. 2Cardiovascular and Metabolic Disorders Programme, Duke-NUS Medical School, Singapore. 3Yong Loo Lin School of Medicine, National University of Singapore, Singapore. 5The Hatter Cardiovascular Institute, University College London, London, UK. 6Cardiovascular Research Center, College of Medical and Health Sciences, Asia University, Taiwan
Shuo Cong and Xavier Chan contributed equally to this article.
Corresponding author:
Professor Derek J. Hausenloy
Email: derek.hausenloy@duke-nus.edu.sg
Handling editor of this article:
Cesario V. Borlongan

In a new window | Download PPT
Figure 1: Summary of potential mechanistic actions of SGLT2 inhibitors.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10835 | 152 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA