International bi-monthly journal of cell signaling, tissue protection, and translational research.
Alpha-synuclein as a Biomarker for Central Nervous System Diseases
Yuning Li1, Mengyuan Guo1, Jia Liu1
Author Affiliations


- 1Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Capital Medical University, Beijing, China.
Abstract
Central nervous system diseases are characterized by slow onset, occultation, and progressive aggravation, which makes the diagnosis of these diseases very difficult. Moreover, many diseases can only be identified at autopsy, so more effective methods are needed for early and differential diagnosis. Alpha-synuclein (α-syn) is a protein widely expressed in the central nervous system. In the physiological state it exists mainly as a monomer. In the pathological state α-syn changes from a soluble monomer to pathological oligomers and fibrils, which participate in the occurrence and development of various central nervous system diseases, including Parkinson's disease (PD), Lewy body dementia, and cerebrovascular diseases. In addition, various forms of α-syn can be transmitted through different body fluids, raising the possibility that it can be used as a biomarker to help diagnose central nervous system diseases. This review explains the physiological functions of α-syn, the effects of different forms of pathological α-syn, summarizes the research progress relating to α-syn in different forms and sources as biomarkers for PD, and explores the potential role of α-syn in other central nervous system diseases and the possibility of α-syn as a biomarker for these diseases. We also propose potential clinical applications of α-syn as helpful biomarkers or therapeutic targets in different central nervous system diseases.
Keywords: Alpha-synuclein, Biomarker, Oligomeric-alpha-synuclein, Parkinson's disease, Phosphorylated alpha-synuclein
Abstract
Central nervous system diseases are characterized by slow onset, occultation, and progressive aggravation, which makes the diagnosis of these diseases very difficult. Moreover, many diseases can only be identified at autopsy, so more effective methods are needed for early and differential diagnosis. Alpha-synuclein (α-syn) is a protein widely expressed in the central nervous system. In the physiological state it exists mainly as a monomer. In the pathological state α-syn changes from a soluble monomer to pathological oligomers and fibrils, which participate in the occurrence and development of various central nervous system diseases, including Parkinson's disease (PD), Lewy body dementia, and cerebrovascular diseases. In addition, various forms of α-syn can be transmitted through different body fluids, raising the possibility that it can be used as a biomarker to help diagnose central nervous system diseases. This review explains the physiological functions of α-syn, the effects of different forms of pathological α-syn, summarizes the research progress relating to α-syn in different forms and sources as biomarkers for PD, and explores the potential role of α-syn in other central nervous system diseases and the possibility of α-syn as a biomarker for these diseases. We also propose potential clinical applications of α-syn as helpful biomarkers or therapeutic targets in different central nervous system diseases.
Keywords: Alpha-synuclein, Biomarker, Oligomeric-alpha-synuclein, Parkinson's disease, Phosphorylated alpha-synuclein
Introduction
Alpha-synuclein (α-syn) is a small molecule protein that is widely expressed in the central nervous system. It exists mainly as a soluble monomer in its normal state and has powerful physiological functions, including regulation of neurotransmission (Levigoureux et al., 2019), synaptic plasticity, and vesicle release (Spillantini et al., 1998). Compared with its physiological function, the pathological function of α-syn has drawn more attention. In the pathological state, α-syn changes from a soluble monomer to pathological oligomers and fibrils, thus participating in the occurrence and development of various central nervous system diseases, such as Parkinson's disease (PD) and Lewy body dementia (DLB) (Burré et al., 2015; Alam et al., 2019; Mehra et al., 2019). In addition, α-syn aggregates are hypothesized to act like prions, inducing transmission of α-syn pathology across different brain regions and even between different tissues (Desplats et al., 2009; Hansen et al., 2011; Volpicelli-Daley et al., 2011); Tran et al., 2014). Precisely because of the transmission properties of pathological α-syn, it has also been detected in some body fluids, such as cerebrospinal fluid (CSF), blood, and tears, suggesting the possibility that it can be used as a biomarker for disease diagnosis (El-Agnaf et al., 2006; Lee et al., 2006; Tokuda et al., 2006; Mollenhauer et al., 2011; Hong et al., 2021).
Biomarkers are the focus of attempts at early diagnosis of central nervous system diseases (Jeromin and Bowser, 2017; van den Berg et al., 2020; Gratpain et al., 2021. As most central nervous system diseases have the characteristics of slow onset, occultation, and progressive aggravation, such diseases have often entered the middle and late stages by the time of clinical diagnosis, which increases the difficulty of disease intervention. For example, the current diagnosis of PD often relies on motor symptoms (Lang and Lozano, 1998a; 1998b). However, motor symptoms occur only when more than 75% of dopaminergic neurons in the substantia nigra have degenerated, which has a negative impact on quality of life (Pang et al., 2022). Therefore, more effective methods for early diagnosis and intervention are needed. Due to the transmissibility of pathological α-syn, detection of various forms of α-syn in CSF, blood, and other body fluids is an important and potential early diagnostic method. Currently, different forms of α-syn are used as biomarkers, including total α-syn, phosphorylated α-syn at Ser129 (p-α-syn), and oligomerized α-syn. These biomarkers have been most studied in the early diagnosis of PD (Pang et al., 2022). However, in recent years, α-syn pathological changes have been thought to be involved in many other central nervous system diseases besides PD. Therefore, different forms of α-syn can be used as biomarkers for diseases, and what role they play in the differential diagnosis of diseases is worthy of further investigation.
In this review, we introduce the physiological functions of α-syn and the effects of these different forms, summarize the research progress of different forms and sources of α-syn as biomarkers for PD, and explore the potential role of α-syn in other central nervous system diseases and the further possibility of α-syn as a biomarker.
Pathophysiology of α-syn
α-Syn
α-Syn, β-syn, and γ-syn belong to the synuclein family and are highly expressed in the human brain (Goedert, 2001; Marques and Outeiro, 2012). These three forms of synucleins are 55-62% identical in sequence and have similar domain organization (Lang and Lozano, 1998b). In addition, α-syn and β-syn have the same subcellular distribution at the presynaptic terminal of neurons (Jakes et al., 1994; Lang and Lozano, 1998b). However, α-syn is the only one in the synuclein family of proteins that is found in dementia with Lewy bodies (DLB) and is thought to be associated with PD pathogenesis (Spira et al., 2001; Spillantini et al., 1997). α-Syn is a soluble cytoplasmic small protein composed of 140 amino acids, which is encoded by the SNCA gene. Its major protein domains include an amphiphilic region, a non-amyloid-β component (NAC) domain, and an acidic tail (Maries et al., 2003; Venda et al., 2010).
The normal physiological function of α-syn has not yet been determined. However, studies with α-syn knockout mice suggest that α-syn may interact with synaptic vesicles and SNARE complex proteins, potentially mediating the transport of synaptic vesicles to, and docking with, the presynaptic membrane, and participating in the regulation of synaptic vesicle dynamics of nerve endings (Cabin et al., 2002). In addition, α-syn knockout mice have shown functional deficits in the dopamine system, suggesting that α-syn may play a role in dopamine neurotransmission (Ghosh et al., 2017). Also, α-syn expression in the yeast model induces calcium signaling disruption and cell death (Callewaert et al., 2020). However, the specific physiological function of α-syn is currently not fully understood, but its pathological function has been examined more and widely studied because its abnormal changes are involved in the occurrence and development of a variety of neurological diseases.
Currently, pathological α-syn is widely studied in PD. Several dominant single-point mutations in the SNCA gene have been found in families with early-onset PD (Polymeropoulos et al., 1997; Lesage et al., 2013). Triploid SNCA genes (Singleton et al., 2003; Farrer et al., 2004) and SNCA polymorphism (Satake et al., 2009; Simón-Sánchez et al., 2009) can induce the disease. Moreover it has been demonstrated that a high level of α-syn in patients is directly associated with cognitive decline, motor, and non-motor symptoms, as well as the severity of neurodegenerative phenotypes (Benskey et al., 2016). In vivo experiments have also demonstrated that drosophila expresses normal and mutant forms of α-syn, and involves dopaminergic neuron loss, filament-containing inclusion bodies, and motor dysfunction (Feany and Bender, 2000; Dabool et al., 2019). Overexpression and aggregation of α-syn in zebrafish models showed decreased mitochondrial activity and increased reactive oxygen species (ROS), which led to neuronal apoptosis and cell death (Robea et al., 2020). Overexpression of the human mutant α-syn leads to the death of dopamine neurons in primary rat embryos (Zhou et al., 2000). Yamada et al. (2004) used a recombinant adeno-associated virus vector (rAAV) system to transfer human α-syn genes into the substantia nigra of rats and observed the loss of approximately 50% of dopaminergic neurons 13 weeks after infection 2004). These results suggest that α-syn dysfunction and accumulation play a key role in PD, which can occur in the axon (Braak et al., 1999) and synapses (Kramer and Schulz-Schaeffer, 2007). These pathogenic accumulations are likely to be key causes of PD and other synucleinopathies.
In addition to PD, α-syn has been implicated in other central nervous system diseases, such as dementia with Lewy bodies (DLB) (Outeiro et al., 2019) and Alzheimer's disease (AD) (Wakabayashi et al., 1998; Monge-García et al., 2021). These are the classic α-syn-related diseases. At the same time, current research indicates that α-syn alterations are also involved in cerebrovascular disease (Chiasserini et al., 2017) and depression (Bruno et al., 2021).
Phosphorylation of α-syn
Post-translational modification, such as phosphorylation, ubiquitination, nitrification, truncation, and oxidation, may promote α-syn misfolding and induce synucleinopathies. This is especially true with phosphorylation (Schmid et al., 2013). Phosphorylated α-syn (p-α-syn) is the main pathological modification form. All phosphorylated α-syn sites, such as Serine 87 (Ser87) (Paleologou et al., 2010; Ha et al., 2014), Serine 129 (Ser129) (Fujiwara et al., 2002; Elfarrash et al., 2021), and tyrosine 39 (Y39) (Zhao et al., 2020b), may cause neuronal toxicity, of which Ser129 is perhaps the most important and most studied. P-α-syn is normally at low levels, whereas more than 90% of α-syn in Lewy bodies is phosphorylated at Ser129 (p-α-syn) (Fujiwara et al., 2002; Elfarrash et al., 2021). Fujiwara et al. (2002) demonstrated that p-α-syn induced insoluble deposition in mouse models was approximately four times higher than for unphosphorylated α-syn (Fujiwara et al., 2002). Meanwhile, Lee et al. (2011) demonstrated that protein phosphatase 2A (PP2A) significantly reduced α-syn phosphorylation at Ser129 and its aggregation in the brain. P-α-syn and its induced aggregation promote the degeneration of dopaminergic neurons (Sato et al., 2011) and can induce Lewy body formation by interacting with ubiquitin (Liu et al., 2007). Mutant S129A, which simulates phosphorylation, enhances α-syn-induced toxicity and behavioral defects in an inherited PD rat model (Gorbatyuk et al., 2008; Azeredo da Silveira et al., 2009). Therefore, phosphorylation of α-syn can be considered to promote the pathogenesis of the disease.
α-Syn in aggregated form
Although some researchers now believe that the natural structure of α-syn is a helix-rich tetramer (Bartels et al., 2011), more studies have shown that the natural structure of most α-syn in its physiological state is unfolded (Chandra et al., 2003). Furthermore, a dynamic equilibrium of unfolded and oligomeric α-syn may exist naturally (Burré et al., 2014; Du et al., 2020). When this equilibrium is disturbed, α-syn monomers form soluble unstable oligomers (Miraglia et al., 2018), and some of the oligomers can further irreversibly form insoluble amyloid fibrils (Ghosh et al., 2015; Du et al., 2020).
Some α-syn oligomers further form amyloid fibrils, forming helix-rich and cytotoxic intermediates that continue to promote α-syn aggregation until the helix-rich oligomers convert to fibrils (Ghosh et al., 2015), with decreasing α-helix secondary structure and increasing β-folding (Apetri et al., 2006). The β-folded structure is the core of α-syn fibrils, and it has been shown that α-syn fibrils are either straight or twisted (Vilar et al., 2008), and have some differences in chirality and length (full-length versus truncated fibrils), which may be due to fibrillary polymorphism (Li et al., 2018). In addition, familial PD mutations result in different fibrillary polymorphisms, suggesting that gene mutations can indeed lead to the formation of different fibrillary structures (Heise et al., 2005; Scott et al., 2010).
Studies have shown that oligomers formed in the initial aggregation stage are effective neurotoxic substances, leading to cell death (Alam et al., 2019) In vitro studies have shown that α-syn oligomers can disrupt mitochondria (Hsu et al., 2000), induce lysosomal leakage (Hashimoto et al., 2004), and destroy microtubules (Alim et al., 2004), which can cause toxicity or interfere with axonal transport of synaptic proteins such as Synapsin-1, leading to synaptic dysfunction and ultimately neurodegeneration (Scott et al., 2010). Mutations that block α-syn oligomerization and fibrillary formation (S87E) induce a significant reduction in α-syn aggregation, fibropathology, and dopaminergic cell loss in the midbrain of rats (Oueslati et al., 2012). These results suggest that the formation of α-syn oligomers may contribute to the pathogenesis of PD and other synucleinopathy. Not only do α-syn oligomers cause toxicity and contribute to the pathogenesis of PD, but α-syn fibrils also play a key role in the transmission of disease pathology in the brain of PD patients (Desplats et al., 2009; Volpicelli-Daley et al., 2011; Recasens and Dehay, 2014; Ghosh et al., 2017). Several researchers have reported that α-syn fibrils have prion-like behavior, meaning they are transmissible. In fact, many studies have shown that exposure to prefabricated fibrils (PFF) leads to aggregation of endogenous α-syn and the formation of LB-like inclusions, which suggests that they act as "seeds" to promote endogenous α-syn accumulation in adjacent neurons after release (Hansen et al., 2011; Tran et al., 2014). Meanwhile, aggregated α-syn has been shown to be released by non-specific pathways including cell death pathways or by specific cellular pathways, and the release of α-syn depends on its folding state under various stress conditions. However, it should be noted that the amyloid fiber properties of these released substances are not clear, and the released species may be non-amyloid oligomers (Karpowicz et al., 2019). Thus, the release of folded α-syn into body fluids may further induce disease progression and facilitate development of the disease.
α-Syn of different forms and sources as biomarkers for PD
In the previous section, as shown in Figure 1, we noted that α-syn monomers, phosphorylated α-syn, and aggregated α-syn all play roles in the pathogenesis of the disease, and specifically the pathogenesis of PD is closely related to the pathological accumulation of α-syn (Bibl et al., 2010; Melki, 2015; Benskey et al., 2016; Candelise et al., 2019; Bassil et al., 2020). Meanwhile, total α-syn, p-α-syn, and α-syn oligomers can be detected in a variety of body fluids, including CSF, blood, and saliva, all of which have potential as biomarkers for early diagnosis of disease. In the following section, we explore the research progress relating to different forms of α-syn from different sources as biomarkers for PD, which is summarized in Table 1.
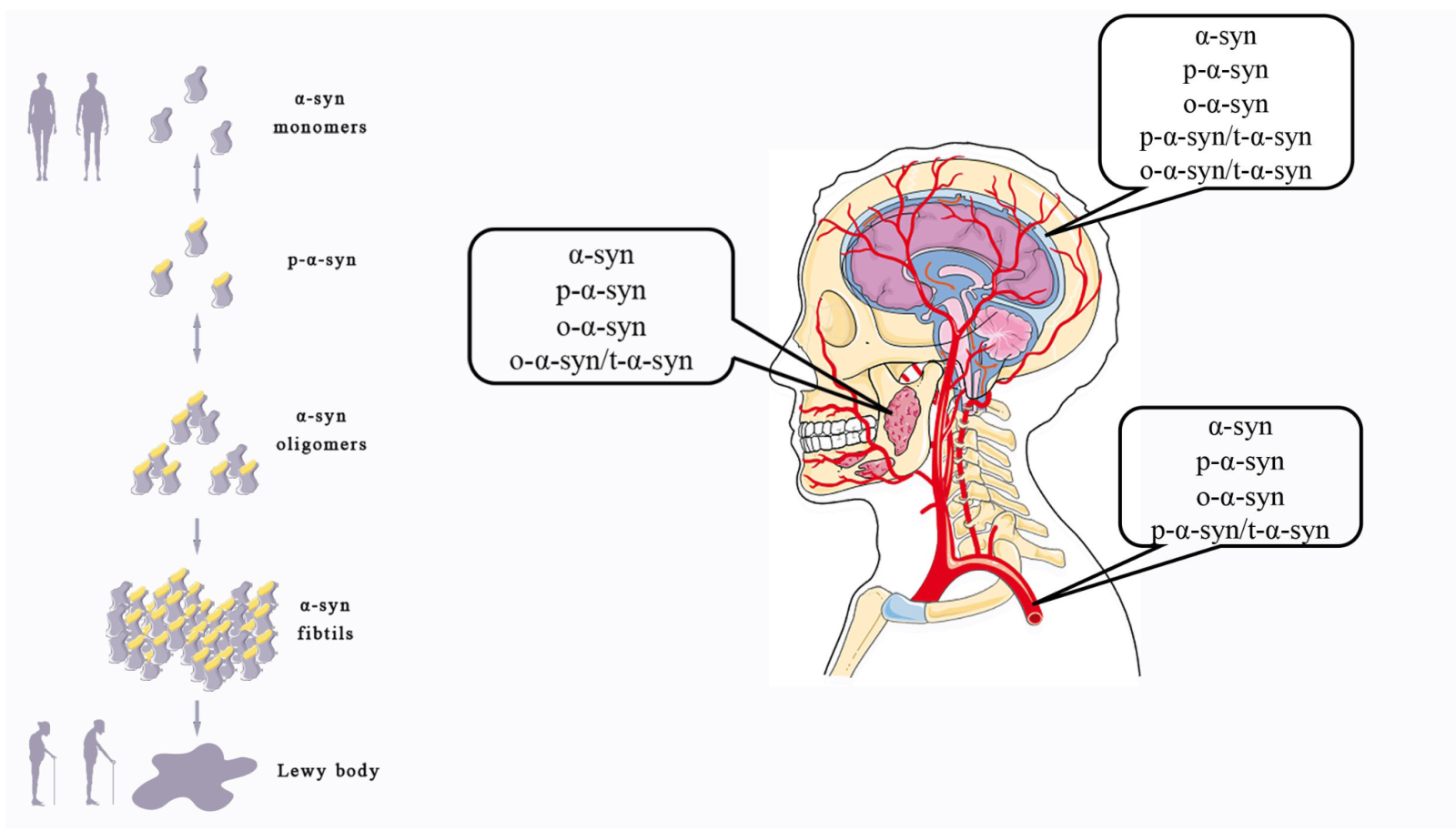
In a new window | Download PPT
Figure 1: Different forms of α-syn take part in the occurrence of PD and can be detected in some body fluids. α-Syn changes from soluble monomesr to pathological oligomers and fibrils, which can promote the onset of PD. Different forms of α-syn can be detected in multiple body fluids.
Total α-Syn
Cerebrospinal fluid
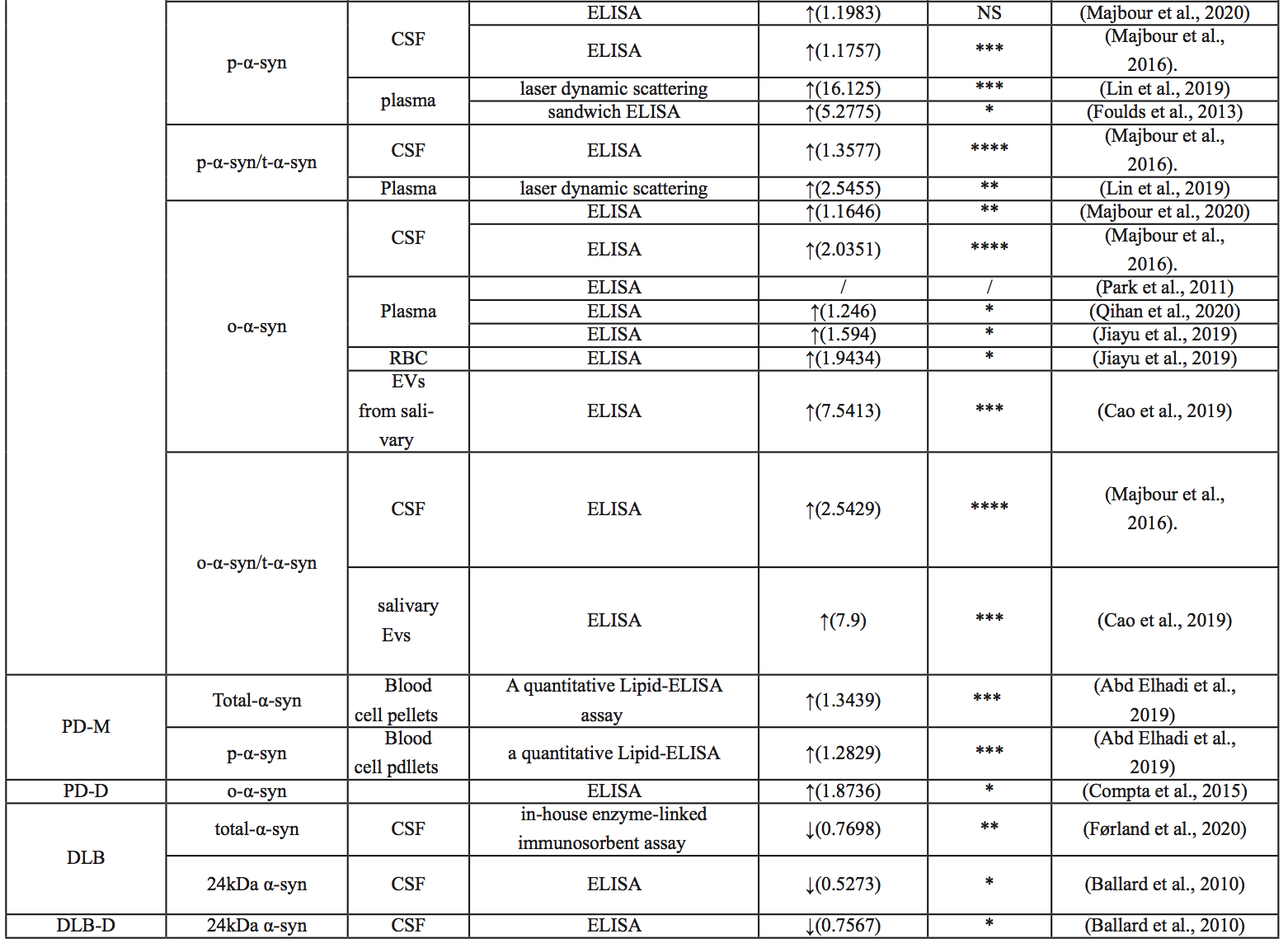
Mollenhauer et al. (2011) examined 273 samples in 2011 and found significant differences in total α-syn in the CSF between the PD and AD groups (P =0.0002), but no significant differences between the DLB and AD groups (P =0.0912). The sensitivity and specificity were 70.72% and 52.83% when the α-syn concentration in CSF was less than 1.6 pg/μl (Mollenhauer et al., 2011). Lee et al. (2006) showed higher α-syn concentrations in the CSF of PD patients than in healthy subjects. However, recent studies have shown that the concentration of α-syn in the CSF of PD patients is lower than that of healthy subjects (van Dijk et al., 2014; Stav et al., 2015; Goldman et al., 2018). Similarly, Førland et al. (2020) showed no significant difference in t-α-syn levels in CSF between PD, DLB, and AD. Total α-syn levels in the CSF were significantly lower in PD patients (434 pg/ml, 16% lower, P = 0.036) than in the control group (Førland et al., 2020). Similarly, Majbour et al. (2020) found a reduced level of t-α-syn in CSF in patients with sporadic PD compared with healthy controls. Majbour et al. (2016) demonstrated a significant decrease in t-α-syn in CSF in PD compared with healthy controls (P < 0.0001).
Blood
Goldman et al. (2018) demonstrated no difference in plasma α-syn concentrations between PD patients and healthy controls. However, other studies have shown increased levels of total α-syn and α-syn hemoglobin complex in blood or plasma in PD patients compared with controls (Abd Elhadi et al., 2019; Yang et al., 2020). When PD patients were subdivided into PD patients with motor symptoms and PD patients with dementia, the concentration of α-syn in the blood of PD patients with motor symptoms was significantly higher than that of healthy people and correlated with the severity of the disease, and the concentration of α-syn in the blood of PD patients with dementia after PD was lower than that of PD patients with motor symptoms (Abd Elhadi et al., 2019). Shi and colleagues (2014) found that total α-syn levels in plasma exosomes were higher in patients with PD than in the healthy control group. However, Zhao et al. (2020a) found that unmodified α-syn showed similar levels of unmodified α-syn in both the PD and control groups.
Saliva
Cao et al. (2020) found that total α-syn levels in salivary extracellular vesicles (EV) in patients with multisystem atrophy (MSA) were lower than those in patients with PD. When t-α-syn is 4.46 pg/ng, multisystem atrophy-Parkinson (MSA-P) can be distinguished from PD, and the area under the curve (AUC) is 0.804 (Cao et al., 2020) In 2018, Goldman et al. (2018) found little difference in the total amount of α-syn in saliva between PD patients and healthy subjects.
Ser129 α-syn
Cerebrospinal fluid
Majbour et al. (2016) demonstrated elevated CSF p-α-syn levels in PD compared with healthy controls, but the difference was not statistically significant. P-α-syn levels in the CSF were not associated with disease severity (Majbour et al., 2016). In 2020, it was found that the CSF of sporadic PD patients was higher than that of healthy people (Majbour et al., 2020). When comparing p-α-syn/t-α-syn ratios, Constantinides et al. (2021) examined CSF from 135 patients, and patients with synucleinopathies (PD and MSA) showed higher p-α-syn/t-α-syn ratios than other patients.
Blood
Lin et al. (2019) showed that p-α-syn levels in plasma were significantly higher in PD patients than in controls. Abd Elhadi et al. (2019) showed that blood p-α-syn in PD patients with motor symptoms was significantly higher than that in healthy subjects, and the level was correlated with the severity of the disease. The levels of p-α-syn in the blood of patients with dementia after PD were also lower than those of PD patients with motor symptoms, but there was no significant difference (Abd Elhadi et al., 2019). Foulds and colleagues (2013) found that plasma p-α-syn was higher in PD patients than in controls, but the clinical application of p-α-syn as a biomarker to distinguish PD patients and healthy controls (AUC 0.717) needs improvement (Foulds et al., 2013). Using a more sensitive immunoassay based on immunomagnetic descent (IMR), Lin and colleagues (2019) showed that p-α-syn distinguished patients with PD from controls at an AUC of 0.94. They also showed that p-α-syn levels were positively correlated with the unified Parkinson's Disease Rating Scale (UPDRS) Part III motor score, suggesting a potential role of p-α-syn in disease severity indices (Lin et al., 2019). Chen et al. (2021) showed that the AUC of serum p-α-syn was 0.92, which predicted the early stages of PD. It should be noted that the average concentration of p-α-syn in PD was significantly different among the different assays; the reasons for this need further investigation. However, various studies have consistently identified p-α-syn in blood as a potential biomarker for PD detection.
α-Syn oligomers
Several studies have shown that neuronal α-syn aggregates consist of α-syn fibrils and various cell membranes, with α-syn fibrils considered pathological (Tu et al., 1998; Gai et al., 2003; Shahmoradian et al., 2019; Lashuel, 2020; Mahul-Mellier et al., 2020). Moreover, according to Braak's theory, oligomers and fibrils formed at the initial stage of the aggregation process are potent neurotoxic agents leading to PD cell death (Braak et al., 1999; Alam et al., 2019). However, some fibrils depolymerize into stable oligomers in vitro (Cremades et al., 2012) and these oligomers remain in a "nonfibrous" state that do not form fibrils (Ingelsson, 2016). Therefore, α-syn oligomers can be detected in a variety of body fluids and are considered a potential biomarker to aid in the diagnosis of PD.
Cerebrospinal fluid
Several studies have shown increased α-syn oligomer concentration in the CSF of PD patients (Park et al., 2011; Parnetti et al., 2014; Compta et al., 2015). Moreover, Majbour et al. (2016) demonstrated a significant negative correlation between levels of CSF α-syn oligomer and H&Y score.
Blood
Park et al. (2011) showed plasma α-syn oligomer concentrations in PD patients and the control group were not different. But Qihan et al. (2020) and Jiayu et al. (2019) both found α-syn oligomers increased in PD patients’ plasma. At the same time, Jiayu et al. (2019) also found α-syn oligomers increased in PD patients’ red blood cells.
Saliva
Saliva is also a rich source of potential biomarkers for disease detection and has some practical advantages over biological fluids currently used to detect neurodegenerative diseases. Cao et al. (2019) found that PD and healthy controls could be differentiated at a concentration of 2.05 pg/ng (sensitivity 92%, specificity 86%).
α-Syn is associated with other central nervous system diseases
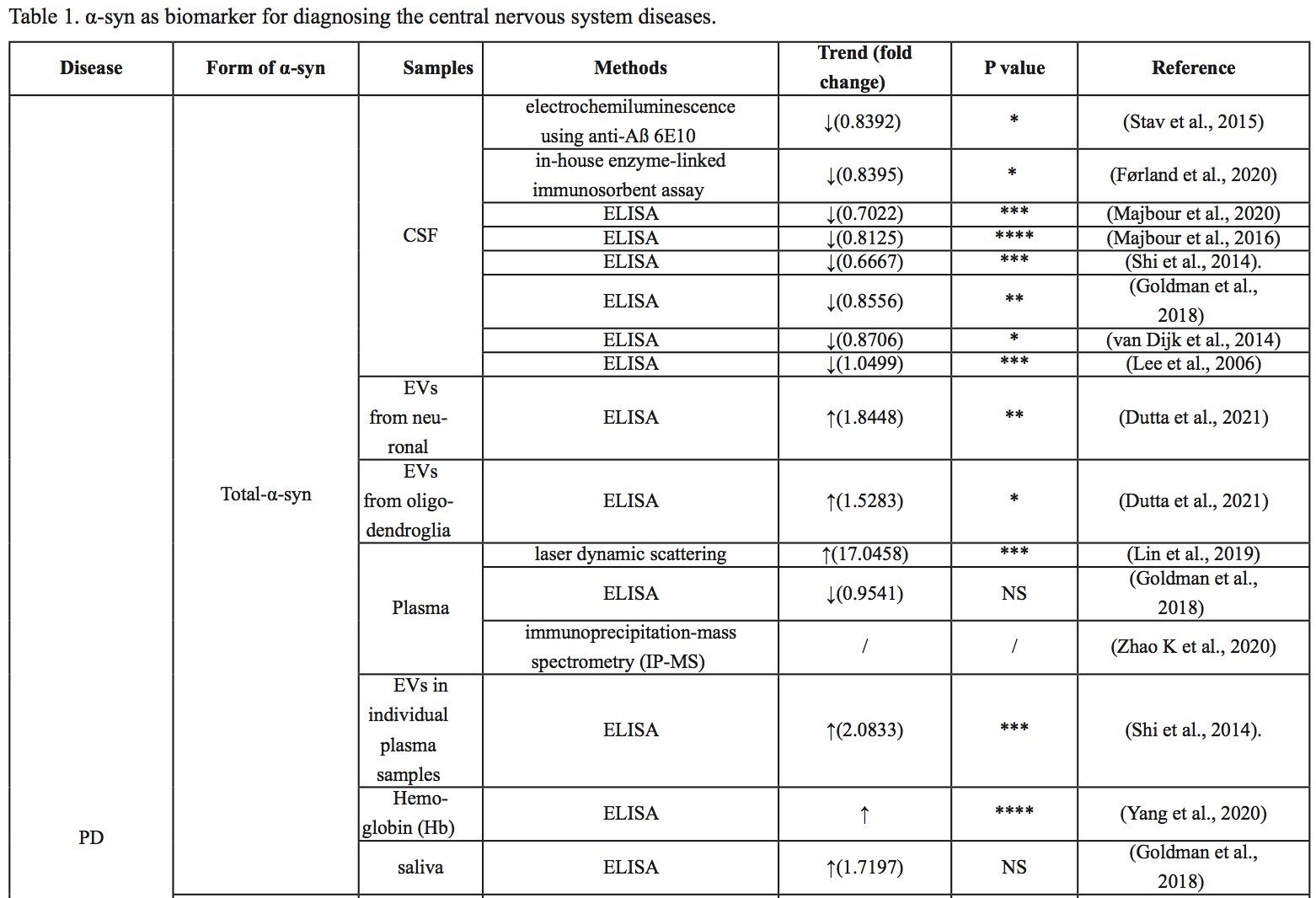
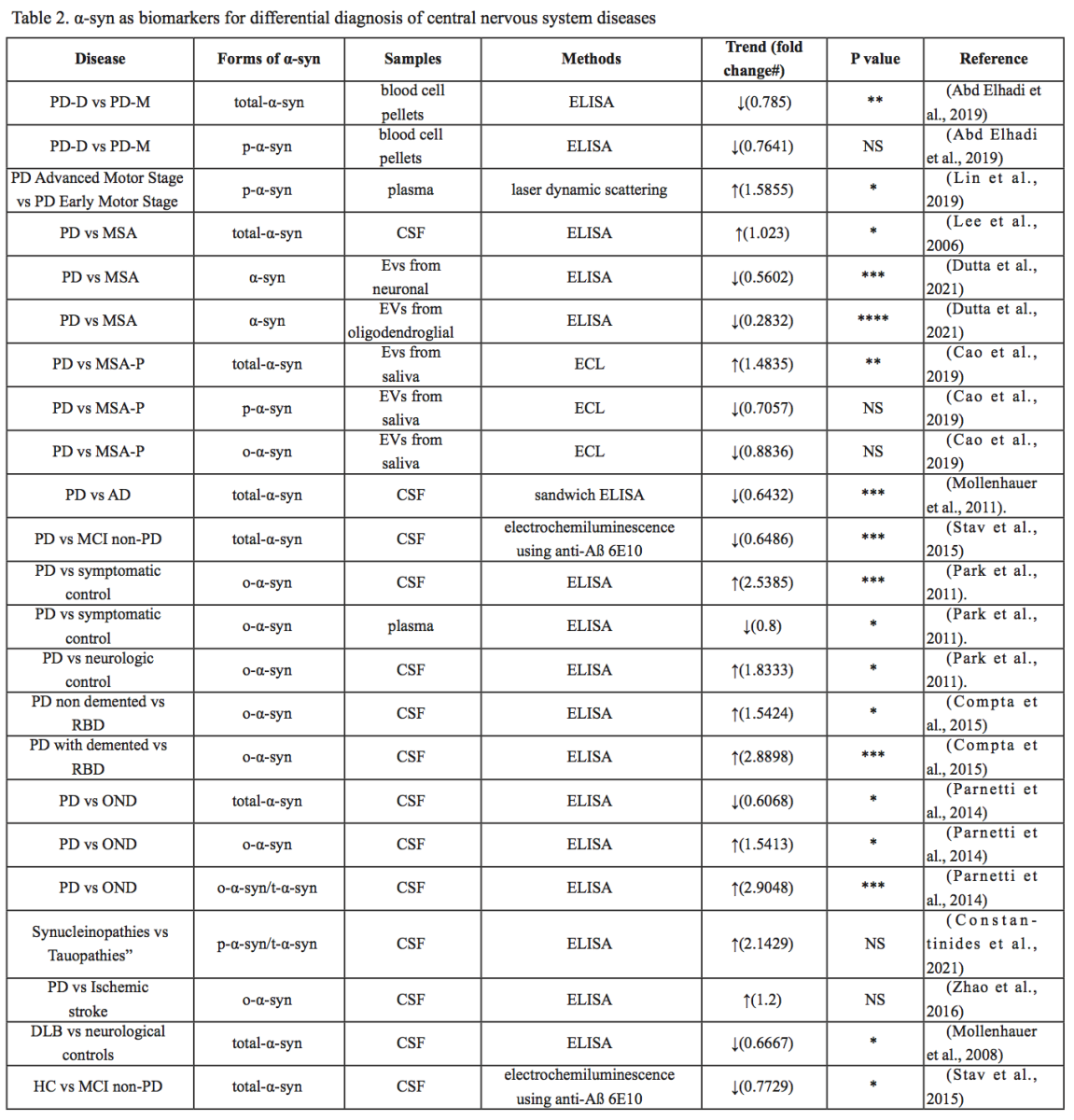
The association between α-syn and PD has been studied extensively, but α-syn has also been implicated in other central nervous system disorders, including DLB, MSA, AD, major depression (MDD), spinal cord injury (SCI), traumatic brain injury (TBI), and ischemic stroke (IS). Differential diagnosis of several diseases is still difficult, and different forms of α-syn from different sources may be used as biomarkers. In the following, we summarize the research progress on the role of α-syn in different diseases, as summarized in Table 1, Table 2 and Figure 2.

In a new window | Download PPT
Figure 2: Different forms of α-syn from different sources may be used as biomarkers to help differentially diagnose several central nervous system diseases.
Dementia with Lewy bodies
DLB is an age-related neurodegenerative disease that causes progressive cognitive decline and interferes with normal living and daily activities. Neuropathologically similar to PD, DLB is characterized by the accumulation of α-syn, which aggregate in Lewy bodies and neuroprocesses in neurons (Pang et al., 2022) (Outeiro et al., 2019). Full-length or near-full-length α-syn has been found in the cingulate cortex and Lewy bodies of DLB patients (Spillantini et al., 1997; Spillantini et al., 1998; Takeda et al., 1998). The deposition of α-syn in Lewy bodies of DLB patients has been shown to be phosphorylated at Ser129 (Obi et al., 2008).
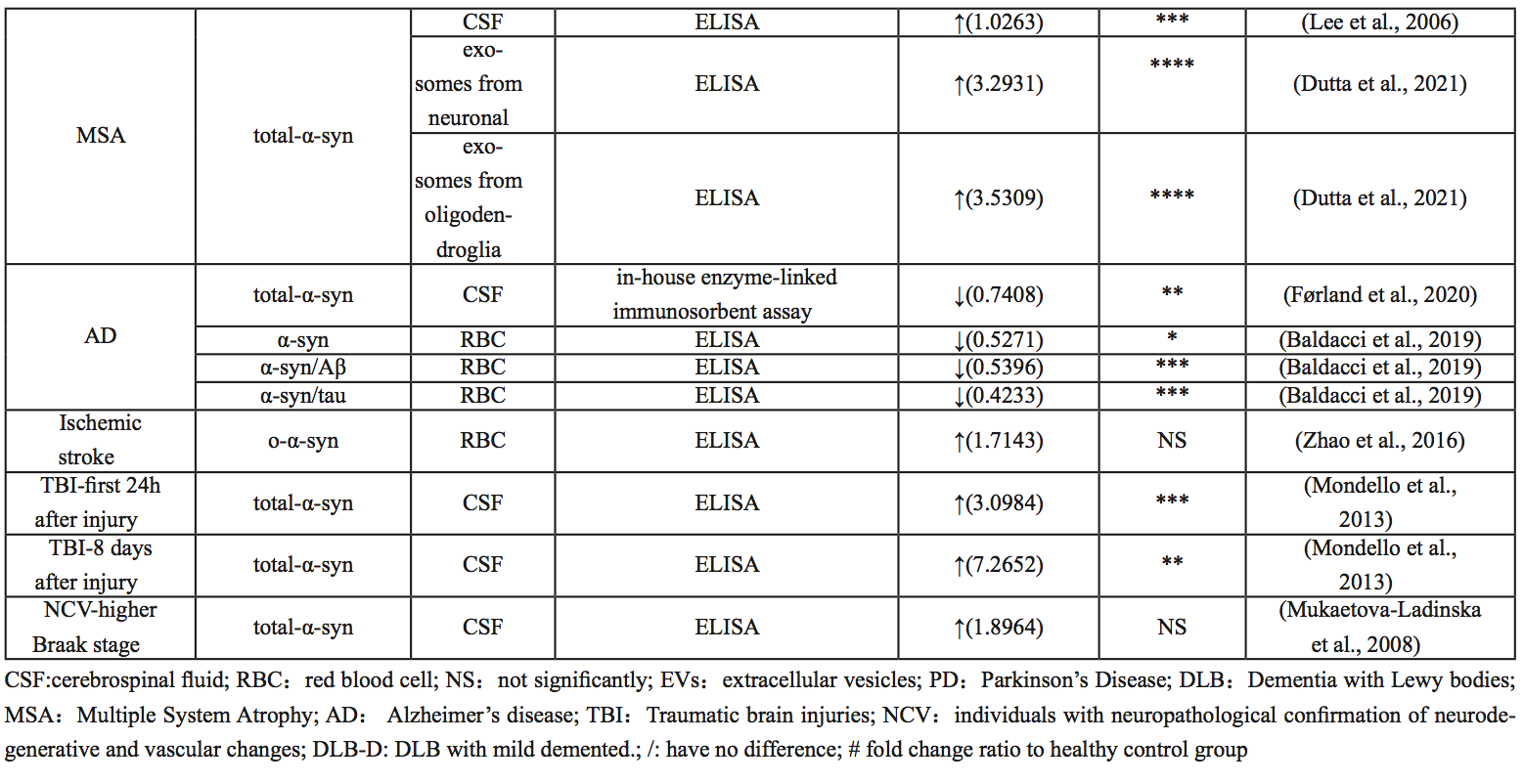
Studies on α-syn levels in CSF are inconsistent. Jakowec et al. (1998) showed that α-syn, a naturally soluble monomer with a molecular size between 14-19kDa, could not be found in CSF. A 2008 study by Mollenhauer et al. (2008), using enzyme-linked immunosorbent assay (ELISA), showed that α-syn levels in CSF in patients with primary synucleinopathies (PD and DLB) were reduced and inversely correlated with age compared with patients with AD or other non-neurodegenerative diseases. However, Mukaetova-Ladinska et al. (2008) measured CSF after death and found increased α-syn concentrations in dementia patients, though this was not statistically significant. DLB patients had lower levels of 24 kDa α-syn in CSF compared to healthy subjects. Moreover, only patients with mild cognitive impairment showed decreased 24 kDa α-syn in their CSF (Ballard et al., 2010), suggesting that 24 kDa α-syn could be used as a biomarker for the severity of cognitive impairment. Interestingly, α-syn has higher fibrosis in the CSF of DLB patients, and the presence of high molecular weight insoluble oligomers has been reported in several studies using Western blotting (Borghi et al., 2000; Compta et al., 2015). In conclusion, different forms of α-syn in CSF may serve as biomarkers to help diagnose DLB.
Multisystem atrophy
MSA is one of a group of synucleinopathies that also includes PD and DLB, and its symptoms often overlap with PD (Pang et al., 2022). In PD and DLB, α-syn accumulates in the Lewy bodies and neuroprocesses in neurons, whereas in MSA, α-syn deposits mainly in oligodendrocytes as glial cytoplasmic inclusions (GCI) (Dutta et al., 2021). Currently, PD and MSA can often only be differentiated at autopsy (Rizzo et al., 2016). However, previous studies have focused on the identification of α-syn as a biomarker and found that α-syn concentrations in oligodendrocyte exosomes were significantly increased compared with healthy controls. In one study, compared with the control and PD group, the concentration of α-syn in neuron exosomes in the MSA group was particularly high (Dutta et al., 2021). In conclusion, the ratio of α-syn concentrations between oligodendrocytes and neuron exosomes can be used as a biomarker to distinguish PD from MSA.
Alzheimer’s disease
AD, the most common cause of dementia, is pathophysiologically characterized by the presence of beta-amyloid plaques and neurofibrillary tangles (Iwai et al., 1995; Scheel et al., 2022). Baldacci et al. (2019) found that patients with AD had lower concentrations of α-syn, α-syn/Aβ, and α-syn/Tau in red blood cells, compared to healthy controls. Although quantitative studies of α-syn protein in CSF of patients with AD are not common, most published results suggest that β-amyloid, Tau, and α-syn can interact with each other and promote aggregation and accumulation among them, accelerating cognitive dysfunction (Clinton et al., 2010; Larson et al., 2012; Korff et al., 2013; Monge-García et al., 2021). CSF α-syn, combined with other AD CSF biomarkers, has clinical value in the differential diagnosis of AD and DLB (Slaets et al., 2014; Berge et al., 2016; Llorens et al., 2016; Chiasserini et al., 2017).
Stroke
Stroke is the most common cerebrovascular disease, which has high morbidity, high mortality, and a high disability rate. Cognitive impairment is one of the major complications after stroke, especially in patients with recurrent ischemic stroke (Pendlebury and Rothwell, 2009; Pang et al., 2022), who are more likely to develop cognitive impairment and dementia (Rost et al., 2022). α-Syn, as a neurodegenerative related protein, is considered to be involved in secondary brain injury after stroke (Liu et al., 2008; Chelluboina et al., 2020; Kim et al., 2021). Studies have also shown that small vascular disease is the most common vascular abnormality in patients with PD, and these vascular abnormalities seem to predispose patients to cerebrovascular injury (Huang et al., 2013).
Several studies have shown that inhibition of α-syn can significantly reduce ischemia-reperfusion induced brain injury and improve neurological function, suggesting that α-syn plays an important role in secondary brain injury after stroke and is a potential target for stroke treatment (Huang et al., 2013; Lohmann et al., 2022). At the same time, studies have shown that α-syn and p-α-syn levels are significantly increased and deposited in neurons in animal models of ischemia-reperfusion, accompanied by mitochondrial damage, oxidative stress, autophagy disorders, increased apoptosis, and loss of dopaminergic neurons in the substantia nigra (Kim et al., 2016; Lohmann et al., 2022). Similarly, clinical studies have shown that the level of α-syn in the red blood cells of stroke patients is significantly higher than that of the normal population, even higher than that of PD patients, and it mainly exists in the form of oligomers (Zhao et al., 2016). Therefore, it is believed that α-syn can be used in the clinical diagnosis of ischemic stroke.
Major depression disorder (MDD)
MDD is a common, chronic, and recurrent mental disorder with high incidence, recurrence, and disability (Crider et al., 2018). Studies have shown that the risk of depression is determined by genes and environment (Penner-Goeke and Binder, 2019). An increasing number of studies have found that the gene SNCA encoding α-syn not only affects the development of neurodegenerative diseases, but also plays a role in the development of mood disorders such as depression. AAV-mediated SNCA overexpression in dopamine neurons not only leads to PD-like dyskinesia but is also associated with mood disorders including depression-like behaviors (Alvarsson et al., 2016; Miquel-Rio et al., 2022). In addition, studies have found that people with major depression have a higher risk of dementia in old age, and synaptic dysfunction in people with major depression may be associated with elevated α-syn levels (Bruno et al., 2021).
Spinal cord injury (SCI)
Spinal cord injury (SCI) is a serious injury of the spinal cord central nervous system, involving multiple damage to tissue structure and dysfunction, resulting in varying degrees of impairment of sensory and motor functions. The disease can lead to permanent disability and is accompanied by sudden autonomic dysfunction such as orthostatic hypotension, a surge in autonomic reflexes and sympathetic activity, and bladder, rectal and sexual dysfunction (Ahuja et al., 2017). SCI is often accompanied by AD, whose pathogenesis is still unclear, but may be related to selective α-syn involvement in autonomic neurons (Zeng et al., 2020).The results showed that α-syn and p-α-syn were significantly increased in the early stage of SCI, and differential expression was mainly concentrated in the white matter of the spinal cord, presenting a spot-like distribution pattern (Zeng et al., 2019a; 2019b). The pathology of α-syn has been shown to spread from the gut to the brain, worsening the surrounding autonomic and somatic nervous systems (Kim et al., 2019). A large number of α-syn aggregates have been detected in the intestinal autonomic nerves of SCI patients, further suggesting that α-syn may be transmitted in the weak autonomic nerves with myelin sheath (Crider et al., 2018). Therefore, its concentration may help in diagnosis.
Traumatic brain injury (TBI)
Traumatic brain injury (TBI) is one of the leading causes of death among young people in developed countries. Similarly to SCI, the initial injury of TBI is caused by mechanical factors that damage the nerve tissue (Drieu et al., 2022). Subsequent lesions may occur within minutes of the injury and may develop for months or even years and may increase the risk of some neurodegenerative diseases (Genrikhs et al., 2017). The large increase in α-syn in CSF may indicate extensive neurodegenerative changes and reflect secondary neuropathological events that occur after injury (Harding and Robertson, 2013; Mondello et al., 2013). In a study on the feasibility of α-syn as an objective biomarker for the diagnosis and prognosis of mild TBI, amyloid-β peptide, tau protein, and α-syn were suggested to be involved in downstream events of TBI, inducing idiopathic cascade reactions (Ikonomovic et al., 2019). In patients with severe TBI, it is often difficult to predict survival or long-term prognosis, especially in the first few days after injury, but patients have significantly higher α-syn levels in the first 24 hours after injury than controls. In patients who survive the injury, α-syn levels tend to normalize after three days, while in patients who die, α-syn levels in the CSF remain elevated eight days after the injury (Mondello et al., 2013). Therefore, levels of α-syn in CSF may be a valuable prognostic biomarker of brain injury.
Summary
α-Syn plays a role in the pathogenesis of many diseases. A possible mechanism is that soluble α-syn monomers aggregate and misfold to form oligomers, which then form amyloid fibrils, and eventually Lewy bodies. Abnormal aggregation of α-syn in neurons may indicate the occurrence of PD and other synucleinopathies. However, some important issues need further exploration. First, which form of α-syn is the key factor in disease development remains unclear. Second, α-syn can be detected in blood, CSF, and tears, which may represent different injury mechanisms. However, the correlation between α-syn concentration and disease needs further study. Third, α-syn expression is altered not only in PD but also in other central nervous system diseases such as AD and stroke. Therefore, it is necessary to further clarify the role of various forms of α-syn as biomarkers for disease and how to make a differential diagnosis of disease based on the variation of α-syn concentration in different body fluids. More importantly, researchers should explore whether α-syn can be used as a biomarker for more diseases, such as brain damage secondary to stroke.
Funding
This research was supported by the National Natural Science Foundation of China (Grant number: 32100925), the Beijing Nova Program (Grant number: Z211100002121038).
Conflict of interest statement
The authors declare that they have no competing interests.
References
Yuning Li1
1Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Capital Medical University, Beijing, China.
Mengyuan Guo1
1Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Capital Medical University, Beijing, China.
Jia Liu1
1Beijing Institute of Brain Disorders, Laboratory of Brain Disorders, Ministry of Science and Technology, Collaborative Innovation Center for Brain Disorders, Beijing Advanced Innovation Center for Big Data-based Precision Medicine, Capital Medical University, Beijing, China.
Corresponding author:
Jia Liu
Email: liujia_19901005@163.com

In a new window | Download PPT
Figure 1: Different forms of α-syn take part in the occurrence of PD and can be detected in some body fluids. α-Syn changes from soluble monomesr to pathological oligomers and fibrils, which can promote the onset of PD. Different forms of α-syn can be detected in multiple body fluids.

In a new window | Download PPT
Figure 2: Different forms of α-syn from different sources may be used as biomarkers to help differentially diagnose several central nervous system diseases.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 6130 | 8 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA