Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Update on preconditioning investigation for traumatic brain injury
Time:2023-04-22
Number:8386
Aim Niamnud1, Winna Xia1, Yinghua Jiang1, Yuwen Xiu1, Yingjie Wang1, Charles J Peper1, Xiaoying Wang1,2, Aaron S. Dumont1, Ning Liu1,2
Author Affiliations


- 1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
- 2Neuroscience Program, Tulane Brain Institute, Tulane University, New Orleans, LA, USA.
Conditioning Medicine 2022. 5(6): 198-204.
Abstract
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality worldwide. Though much progress has been made in the field of medicine and technology, there is no effective pharmacological therapy for TBI in the clinic. Preconditioning scenarios can serve as a potential neuroprotective strategy as well as induce tolerance against harmful cascades of cellular events following primary injury. An effective preconditioning strategy against TBI could be groundbreaking for scientists in this field as well as in the clinical setting. This review focuses on the current status of preconditioning methods in TBI studies and elucidating the mechanisms underlying the beneficial effects of various preconditioning scenarios. Further preconditioning studies are needed to aid in understanding the molecular pathological mechanisms of brain damage and recovery as well as developing novel and effective intervention strategies for TBI.
Keywords: Preconditioning, Traumatic brain injury, Methods, Mechanisms
Abstract
Traumatic brain injury (TBI) is one of the leading causes of morbidity and mortality worldwide. Though much progress has been made in the field of medicine and technology, there is no effective pharmacological therapy for TBI in the clinic. Preconditioning scenarios can serve as a potential neuroprotective strategy as well as induce tolerance against harmful cascades of cellular events following primary injury. An effective preconditioning strategy against TBI could be groundbreaking for scientists in this field as well as in the clinical setting. This review focuses on the current status of preconditioning methods in TBI studies and elucidating the mechanisms underlying the beneficial effects of various preconditioning scenarios. Further preconditioning studies are needed to aid in understanding the molecular pathological mechanisms of brain damage and recovery as well as developing novel and effective intervention strategies for TBI.
Keywords: Preconditioning, Traumatic brain injury, Methods, Mechanisms
Introduction
Traumatic brain injury (TBI) is a significant cause of death and disability worldwide (Capizzi et al., 2020), but there is currently no effective pharmacological therapy. It is well known that primary mechanical injury leads to focal brain structural damage and diffuse axonal injury, followed by secondary brain injuries, including neuroinflammation, blood brain barrier (BBB) disruption, and neuronal cell death (Monsour et al., 2022). Although many injurious pathways are well defined, it has been a major challenge to identify ways to mitigate these pathways to reduce secondary brain damage after TBI. Preconditioning has been investigated at a basic-science level to determine the endogenous and pluripotent neuroprotective effects, and has shown potential as a new therapy for TBI (Yokobori et al., 2013).
The term “preconditioning” was first introduced by Janoff in 1964 (Janoff, 1964) to describe a phenomenon in which brief episodes of a noxious stimulus with sublethal insult or stress induce certain advantageous intrinsic mechanisms for protection and repair, creating a tolerance in the organ to the insult and, thus, protecting it from subsequent lethal injuries (Janoff, 1964; Dirnagl et al., 2009). Since then, various research on preconditioning has been aimed at understanding endogenous neuroprotective mechanisms and identifying innovative therapeutic strategies that prevent or reduce neuronal damage (Dirnagl and Meisel, 2008; Stetler et al., 2014). The results from preconditioning studies in stroke have been very promising and are now being applied in the clinic (Dirnagl et al., 2009; Hess et al., 2021), but preconditioning investigations in TBI have been limited. The main reason is the majority of TBI that occur are accidental, so previous investigations have focused more on post-TBI intervention and treatment. However, sport and military-related mild TBI or high-risk elective neurosurgical patients, such as aneurysm reconstructions, which occur in a more predictable and orchestrated timeframe (Stetler et al., 2014), make preconditioning interventions and treatment of mild TBI feasible. Importantly, the concept of preconditioning is highly attractive for investigators to study the endogenous molecular mechanisms underlying neuroprotection after TBI elicited by preconditioning, which may help in developing new therapy (Stetler et al., 2014). Effective preconditioning stimuli in TBI are numerous and diverse, ranging from heat acclimation, hypoxia, ischemic, physical exercise, hyperbaric oxygen, and exposure to neurotoxins and pharmacological agents. These preconditioning stimuli are vastly different, but the sublethal exposure of these stimuli can confer protection against TBI through multiple mechanisms, such as activation of extra- and intracellular defense mechanisms, inhibition of neuronal cell death and oxidative stress, reduction in neuroinflammation, and induction of neurogenesis, etc. (Stetler et al., 2014). An improved understanding of preconditioning in TBI would help us identify innovative therapeutic strategies that prevent or at least reduce neuronal damage (Dirnagl and Meisel, 2008).
Previous studies have already summarized TBI pathophysiology and discussed existing animal studies demonstrating the efficacy of preconditioning in TBI (Yokobori et al., 2013; Stetler et al., 2014). In this review, we focused on the current progress in the experimental methods of preconditioning in TBI, and then elucidating the mechanisms underlying preconditioning that triggers endogenous neuroprotection.
Preconditioning methods and mechanisms for TBI
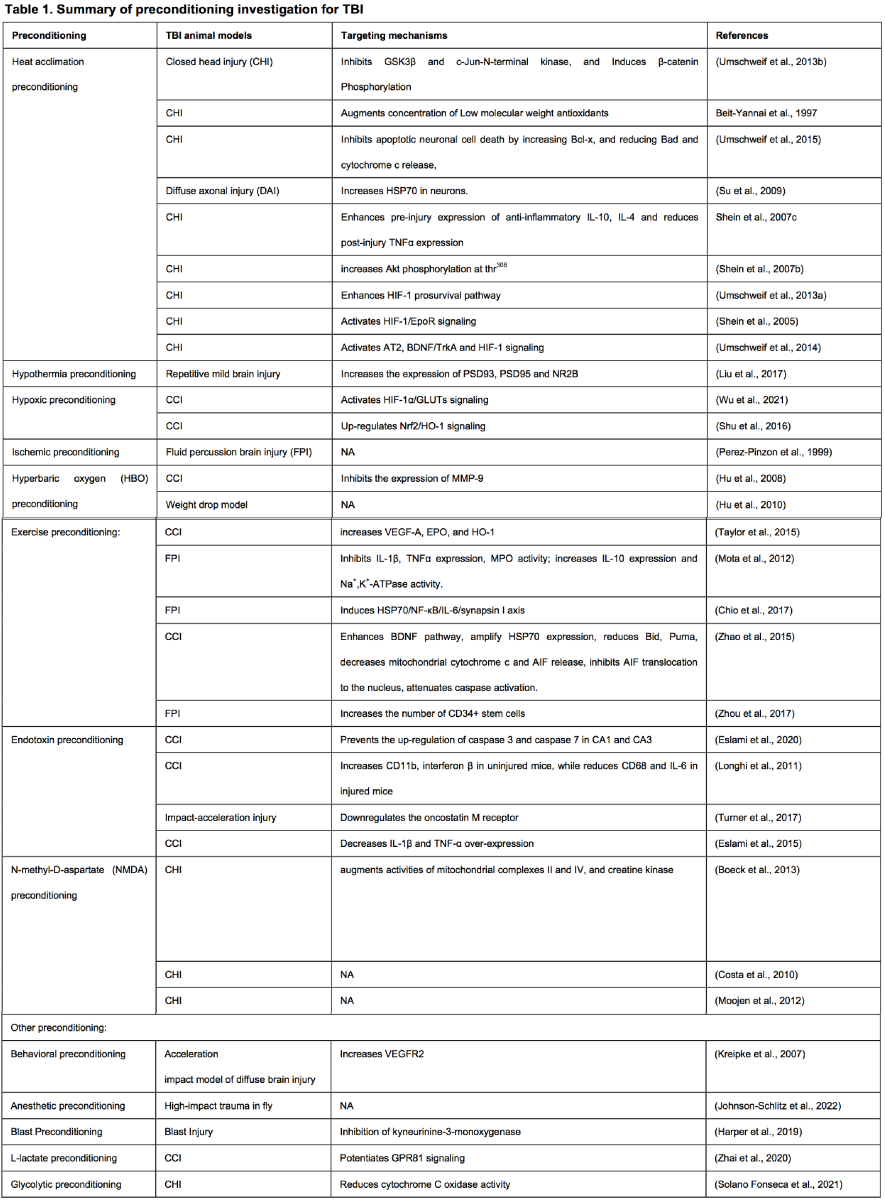
Many deleterious stimuli including heat acclimation, hypoxia, ischemia, hyperbaric oxygen, endotoxin, drugs, and stimuli that essentially induces cellular stress, such as anesthetics, and physical exercise have been reported as preconditioning methods for TBI. Below we summarized these methods and elucidated the associated underlying mechanisms of each stimulus (Figure 1 and Table 1).
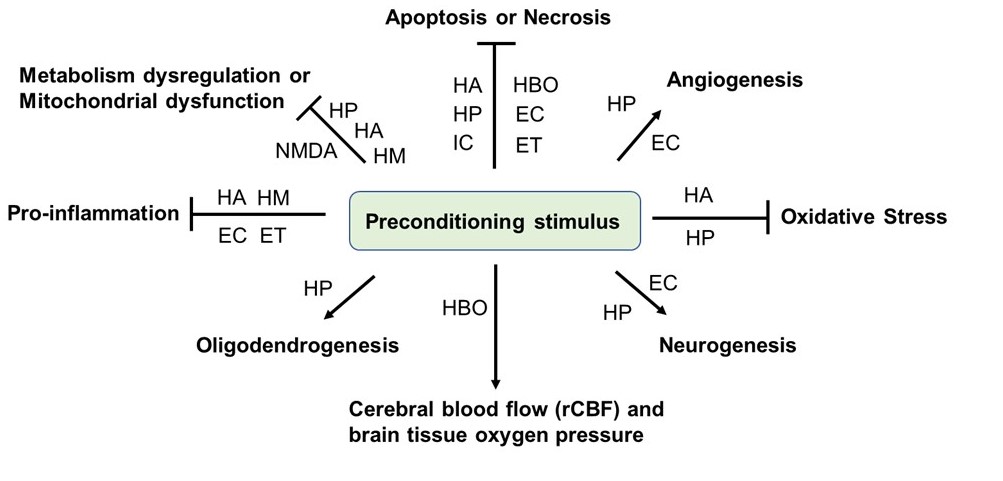
In a new window | Download PPT
Figure 1: Preconditioning-mediated beneficial effects in TBI studies. Animal studies on TBI have demonstrated that various preconditioning stimuli or stresses trigger numerous beneficial effects in TBI animal models, including inhibition of metabolism dysregulation, mitochondrial dysfunction, pro-inflammation, oxidative stress, and neuronal cell death, while promoting neurogenesis, oligodendrogenesis, angiogenesis, cerebral blood flow, and brain tissue oxygen pression. HA: heat acclimatiion; HM: hypothermia; HP: hypoxia; IC: ischemic; HBO: hyperbaric oxygen; EC: exercise; ET: endotoxin; NMDA: N-methyl-D-aspartate.
Temperature preconditioning –Heat acclimation (HA) and hypothermia (HM)
Heat acclimation (HA) (34°C 30 d) is a well-established preconditioning model that confers neuroprotection against experimental closed head injury (CHI) (Beit-Yannai et al., 1997; Umschweif et al., 2013b). Heat acclimation is unique from other preconditioning methods since this global physiological adaptation has been shown to induce cross-tolerance against other stressors, including TBI (Shein et al., 2007a; Horowitz et al., 2015).
The precise mechanisms underlying HA preconditioning-induced neuroprotection against TBI have been thoroughly studied, including reduced basal metabolic rate and improved heat tolerance (Horowitz, 2002), inhibited apoptotic neuronal cell death (Umschweif et al., 2015), activated heat shock response (HSP)(Su et al., 2009), anti-oxidative stress, and anti-inflammation (Shein et al., 2007c; Shein et al., 2007a; Shein et al., 2008), which are mediated by several molecular effectors and signaling cascades. Accumulating evidence suggested that Akt is a key cellular pro-survival signaling pathway that mediates HA-induced neuroprotection. Preconditioning via HA induces neuroprotection by increasing Akt phosphorylation at thr308 (Shein et al., 2007b). Further study suggested that HA mediates neuroprotection by inhibiting Akt downstream at the GSK3β/β-catenin pathway after TBI (Umschweif et al., 2013b). Another key mediator of HA preconditioning-induced neuroprotection in TBI is heat inducible factor-1 alpha (HIF1α). Studies suggested that HA confers neuroprotection against CHI by increasing HIF1α (Shein et al., 2007b; Umschweif et al., 2013a) and its downstream erythropoietin receptor (Shein et al., 2005). Angiotensin II receptor type 2 (AT2) has also been found to be an upstream regulator of neuroprotection and neurorepair in injured HA mice (Umschweif et al., 2014). In addition, microglia-derived brain-derived neurotrophic factor (BDNF) has shown to be capable of mediating HA-conferred neuroprotection following experimental TBI (Shein et al., 2008).
In addition, temperatures below homeostatic temperatures (hypothermia) can induce a tolerant state. Hypothermia has now been applied after TBI in the clinic and in experimental research (Lewis et al., 2017; Hui et al., 2021). However, research on the effect of hypothermia preconditioning on TBI-induced brain damage is scarce. Only one study has reported that hypothermic preconditioning (mice were placed at room temperature for approximately 60 min until the core temperature was lowered to 25 °C Then, mice were transferred to a freezer (5 °C) and the core body temperature of mice decreased to 16–18 °C for 45 min) improves cognitive impairment by enhancing synaptic plasticity, inhibiting microglial activation, and improving hypometabolism in the brains of TBI mice (Liu et al., 2017).
Hypoxic preconditioning
Hypoxic preconditioning is a well-established preconditioning model that increases the inherent tolerance of brain tissue suffering from severe hypoxia or ischemic insult (Stetler et al., 2014; Liu et al., 2021a). Accumulating evidence suggested that hypoxic preconditioning also protects the brain against TBI (Chang et al., 2013; Shu et al., 2016; Wu et al., 2021). For example, hypoxic preconditioning has been reported to protect neuronal cells against CCI in rats through upregulation of HIF-1α/glucose transporters (GLUTs) signaling pathway and stimulation of glucose transport (Wu et al., 2021). Another study suggested that the neuroprotection of hypoxic preconditioning on rat brain against TBI can be ascribed to up-regulation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and hemo ocygenase 1 (HO-1) expression, thereby alleviating oxidative stress damage (Shu et al., 2016). In addition, hypoxic conditioning of stem cells has also shown therapeutic potential against TBI. Previous study suggested that TBI rats treated with hypoxic-preconditioned mesenchymal stem cells (MSC) secretome, which included hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), performed significantly better in both motor and cognitive tasks, showed an increased in neurogenesis, and had significantly less brain damage than the TBI rats treated with the normoxic-preconditioned MSC secretome (Chang et al., 2013). In addition, it has been reported that intracranial transplantation of hypoxic-preconditioned induced pluripotent stem cells (iPSC)-derived neural progenitor cells alleviate neuropsychiatric defects after TBI in juvenile rats (Wei et al., 2016). More recently, hypoxic preconditioning has been reported to enhance the differentiation of bone marrow stromal cells into mature oligodendrocytes to promote remyelination via modulation of the pro-survival mammalian target of rapamycin (mTOR)/HIF-1α/VEGF Yuan et al., 2020).
Ischemic preconditioning
Ischemic preconditioning now has proven efficacy against stroke in numerous animal and clinical studies (Gao et al., 2021; Hess et al., 2021). Moreover, remote ischemic conditioning in trauma patients with severe TBI can significantly decrease secondary brain injury (Joseph et al., 2015). However, only one study employed ischemic preconditioning for TBI, but this approach has shown beneficial effects. Research has demonstrated that ischemic preconditioning 48 h prior to fluid-impact brain injury had fewer necrotic cell counts and less contusion volume than the control group (Perez-Pinzon et al., 1999). Therefore, the protective fortification established by ischemic preconditioning may be able to extend to acute brain injury.
Hyperbaric oxygen (HBO) preconditioning
Compared with other preconditioning stimuli, hyperbaric oxygen (HBO) is benign and has clinically translational potential (Hu et al., 2016). Accumulated evidence shows that HBO preconditioning not only reduces ischemic brain injury (Stetler et al., 2014; Liska et al., 2018), but also induces tolerance to TBI (Hu et al., 2008; Hu et al., 2010; Eve et al., 2016). Moreover, it has been suggested that hyperbaric oxygen preconditioning treatment (HBOT) is a potential treatment for post-traumatic stress disorder for individuals that have sustained TBI (Eve et al., 2016). The mechanism of HBO preconditioning-induced neuroprotection, including the inhibitory effect on the expression of matrix metallopeptidase 9 (MMP-9) (Hu et al., 2008), which plays a key role in promoting neuronal cell death (Zhao et al., 2006), and the amelioration of regional cerebral blood flow (rCBF) and brain tissue oxygen pressure (PbtO2) (Hu et al., 2010).
Exercise preconditioning
A considerable set of evidence suggest that exercise preconditioning attenuates neuronal loss, reduces neuroinflammation, and improves neurological outcomes after TBI (Mota et al., 2012; Taylor et al., 2015; Zhao et al., 2015; Chio et al., 2017). Multiple factors have been reported to mediate neuroprotection evoked by exercise preconditioning. It has been reported that exercise preconditioning increases the expression of specific neuroprotective genes and proteins (VEGF-A and erythropoietin [EPO], but not HO-1) in the brain (Taylor et al., 2015). In addition, exercise preconditioning reduces initial damage and limits long-term secondary degeneration after fluid percussion brain injury (FPI) by improving the cerebral inflammatory status profile, including reducing pro-inflammatory cytokines tumour necrosis factor alphas (TNFα) and interleukin (IL)-1 beta, and increasing anti-inflammatory IL-10 in rats (Mota et al., 2012). Moreover, exercise preconditioning has been shown to attenuate neurological deficits by stimulating a critical HSP70/nuclear factor kappa-light-chair-enhancer of activated B cells (NF-κB)/IL-6/synapsin I axis in TBI rats (Chio et al., 2017). Additionally, exercise preconditioning activated multiple antiapoptotic mechanisms, including BDNF and HSP70, reduced,the proapoptotic Bcl-2 family molecules (Bid, Puma), decreased mitochondria permeabilization with the attenuated release of cytochrome c and apoptosis-inducing factor (AIF), reduced AIF translocation to the nucleus, and attenuated caspase activation (Zhao et al., 2015). Recently, cold water swimming (3 minutes at 4°C) preconditioning has been reported to promote the proliferation of endothelial progenitor cells and angiogenesis in peripheral blood and hippocampus, concurrent with amelioration of cognitive deficits caused by the experimental rat model of TBI (Zhou et al., 2017).
Endotoxin preconditioning
Excessive inflammation after TBI can cause secondary neuronal injury and long-term neurological deficits (Liu et al., 2021b). Despite endotoxin lipopolysaccharide (LPS) (Hang et al., 2004) and its toll-like receptors (TLRs) (Jiang et al., 2018) playing detrimental roles in neuroinflammation in TBI, accumulating evidence suggest that endotoxin preconditioning with a low/sublethal dose of LPS prior to TBI provides robust neuroprotection. It has been reported that LPS preconditioning inhibits neural damage and apoptosis induced by trauma in the hippocampus (Eslami et al., 2020). Moreover, the beneficial effects of LPS preconditioning with low doses can last for up to one month after injury, the mechanisms involved might be associated with modulating microglial/macrophage activity and facilitating M2 microglia activation (Longhi et al., 2011). Another study showed that single low-dose LPS preconditioning was neuroprotective in a close-head model of diffuse axonal injury and significantly reduced the post-injury gliosis response near the corpus callosum, possibly by activating TLR4 signaling cascades and downregulating the oncostatin M receptor to suppress innate immunity and astrocyte activation (Turner et al., 2017). Low-dose LPS preconditioning has also been reported to prevent the acceleration of kindling epileptogenesis by decreasing IL-1β and TNF-α over-expression, and the number of damaged neurons in the hippocampus of TBI rats (Eslami et al., 2015).
N-methyl-D-aspartate (NMDA) preconditioning
It is well established that glutamatergic excitotoxicity-induced cellular damage after TBI is mediated by the excitatory neurotransmitters, glutamate and aspartate, through the excessive activation of N-methyl-D-aspartate (NMDA) receptors (Costa et al., 2010; Boeck et al., 2013). Preconditioning with a low dose of NMDA was also used as a strategy for protection against TBI. It has been reported that NMDA preconditioning improves short-term motor deficits after mild TBI in mice (Costa et al., 2010). Subsequently, NMDA preconditioning has been reported to prevent object recognition memory impairment and increase brain viability, inducing impairment of long-term memory in TBI mice (Moojen et al., 2012). Mechanistically, after 24 h, TBI mice preconditioned with NMDA demonstrated augmented activities of complexes II and IV in the cerebral cortex and/or cerebellum, and creatine kinase in the cerebral cortex (Boeck et al., 2013).
Other preconditioning
In addition to the aforementioned preconditioning methods, which had been extensively studied, other preconditioning techniques have also been reported to protect against TBI. These preconditioning methods needs to be further studied and precise mechanisms have yet to be elucidated. For example, behavioral preconditioning consisting of daily exposure of animals to the radial arm maze enhances cognitive outcome after brain trauma. In this study, increased angiogenesis was proposed to underlie the cognitive sparing seen in pre-conditioned animals (Kreipke et al., 2007). Anesthetic preconditioning has been found to confer neuroprotection against TBI. Pre-exposure to isoflurane or sevoflurane preconditioned fed control flies to TBI, reducing the risk of death but had no preconditioning effect in obese flies, suggesting that obesity thwarts the brain-protective effect of anesthetic preconditioning (Johnson-Schlitz et al., 2022). Moreover, a study has found that low-intensity blast preconditioning protects retinal ganglion cells following blast-mediated TBI (Harper et al., 2019). Recent studies suggested that metabolism-associated pathways or molecules can also confer preconditioning against TBI. It has been found that L-lactate preconditioning promotes plasticity-related protein expression and reduces neurological deficits by potentiating G protein-coupled (GPR)-81 signaling in rat TBI models (Zhai et al., 2020). Furthermore, glycolytic preconditioning in astrocytes has also been demonstrated to mitigate neurodegeneration after closed-head TBI in mice (Solano Fonseca et al., 2021). Interestingly, miRNA-21 preconditioning has been proposed to induce tolerance to TBI (Lopez et al., 2017).
Perspective
In this review, we introduced multiple preconditioning experimental methods in the animal TBI model and the mechanisms underlying preconditioning that triggers endogenous protection against TBI. Despite the promising pre-clinical studies outlined above, the translation of evidentiary experimental preconditioning into clinical implementation remains a big challenge.
Based on the preconditioning studies in TBI, multiple TBI animal models, including CCI, CHI, etc. have been employed in the preconditioning studies. The preconditioning effects and underlying neuroprotective mechanisms may be similar but may also be distinct between different TBI models. Notably, the most common pathological mechanisms shared by multiple preconditioning strategies is anti-apoptosis or necrosis, followed by inhibition of metabolic dysregulation or mitochondrial dysfunction, and anti-pro-inflammatory responses (Figure 1). Whether other mechanisms also underlie the beneficial effect of various preconditioning need to be further investigated. It has been discovered that some preconditioning strategies have a relatively short-term effect, as the preconditioning response fades within a week or two (Stetler et al., 2014). Future directions should investigate how to make the preconditioning provide continuous neuroprotection for a long period of time after TBI. Compared to other preconditioning methods, the physical means of preconditioning such as temperature preconditioning, HBO, and exercise may potentially be employed for TBI therapy. This is because they are non-invasive, relatively safe, and especially relevant for the possible clinical implication in specific populations at risk of TBI, such as soldiers, sport combatants, or individuals involved in any high-risk physical activities (Yokobori et al., 2013). However, the results obtained from animal experiments may not be directly applicable to humans, and further comprehensive studies are still required. In addition, the mechanistic studies suggested that multiple pathways, including HIF1, HSP, and TLRs/NF-κB pathways have been found to be the common molecular mechanisms underlying the beneficial effects of multiple preconditioning strategies against different TBI models (Table 1). Importantly, HIF1 and HSP signaling pathways play central roles in cross-tolerance among heat acclimation, hypoxia, and exercise preconditioning approaches (Ely et al., 2014). Therefore, targeting these pathways may be promising to the treatment of TBI in the future. Considering that metabolism dysregulation plays a central pathological role in initiating secondary brain injury after TBI (Brooks and Martin, 2014), and metabolic preconditioning have shown promising effects in TBI animal studies (Zhai et al., 2020; Solano Fonseca et al., 2021), future preconditioning studies by employing metabolic stress may help in investigating metabolism dysregulation-associated endogenous anti-inflammatory and/or neuroprotective mechanisms. Another promising field in the use of preconditioning methods for TBI is to combine the approach with stem cells. It has been well-established that preconditioning strategies can enhance neural stem cell-based therapy for ischemic stroke (Othman and Tan, 2020). Emerging evidence suggest that hypoxic-preconditioned stem cells, including MSC (Chang et al., 2013), hypoxia-preconditioned iPSC-derived neural progenitor cells (Wei et al., 2016), and bone marrow stromal cells (Yuan et al., 2020) have shown promising and beneficial effects in TBI animal studies. Therefore, stress preconditioning of stem cells may lead to innovative and promising new therapy in TBI, however, these preconditioning strategies remain to be further explored.
In summary, this review provided updates on various methods of TBI preconditioning based on current findings on this topic, as well as introduced the underlying mechanisms of some preconditioning methods in TBI. Understanding the means and mechanisms of stimuli that induce these endogenous mechanisms in the brain will aid in the translation of interventions to prevent and/or limit TBI. Substantial work is still required to decipher preconditioning methods and determine the mechanisms involved.
Conflict of interest statement
The authors declare no competing financial interests.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grants (RO1 NS092085 to XW).
References
Aim Niamnud1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Winna Xia1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Yinghua Jiang1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Yuwen Xiu1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Yingjie Wang1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Charles J Peper1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Xiaoying Wang1,2
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA. 2Neuroscience Program, Tulane Brain Institute, Tulane University, New Orleans, LA, USA.
Aaron S. Dumont1
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA.
Ning Liu1,2
1Clinical Neuroscience Research Center, Department of Neurosurgery and Neurology, Tulane University School of Medicine, New Orleans, LA 70112, USA. 2Neuroscience Program, Tulane Brain Institute, Tulane University, New Orleans, LA, USA.
Corresponding author:
Ning Liu
Email: nliu3@tulane.edu

In a new window | Download PPT
Figure 1: Preconditioning-mediated beneficial effects in TBI studies. Animal studies on TBI have demonstrated that various preconditioning stimuli or stresses trigger numerous beneficial effects in TBI animal models, including inhibition of metabolism dysregulation, mitochondrial dysfunction, pro-inflammation, oxidative stress, and neuronal cell death, while promoting neurogenesis, oligodendrogenesis, angiogenesis, cerebral blood flow, and brain tissue oxygen pression. HA: heat acclimatiion; HM: hypothermia; HP: hypoxia; IC: ischemic; HBO: hyperbaric oxygen; EC: exercise; ET: endotoxin; NMDA: N-methyl-D-aspartate.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8386 | 26 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA