Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
PiRNA's significance in CNS disorders and its potential role in conditioning-induced brain adaptation
Time:2024-01-16
Number:5682
Rohan Mahesh Patil1, Ornella Cuomo1, Giuseppe Pignataro1
Author Affiliations


- 1University of Naples “Federico II”, School of Medicine and Surgery, Neuroscience, Napoli, Italy.
Conditioning Medicine 2023. 6(3): 88-97.
Abstract
This literature review focuses on the function of p-element-induced wimpy testis (PIWI)-interacting RNAs (piRNAs) in CNS disorders and their potential role in brain conditioning. While piRNAs have been widely investigated in germ cells, their role in brain function and neurological conditions remains unknown. This review gives an overview of piRNAs, their synthesis, and their interactions with PIWI proteins. It explores the potential function of piRNAs in gene regulation, chromatin remodeling, and interactions with other small RNAs. This review also investigates the role of piRNAs in diverse pathologies such as cancer, cardiovascular disease, reproductive system defects, and neurological diseases. In the setting of neurodegenerative disorders, deregulation of piRNA expression has been linked to Alzheimer's, Parkinson's, and Huntington's diseases, indicating their potential as therapeutic targets. The article continues to look at the role of piRNAs in the central nervous system, including their functions in inflammation, apoptosis, and neurogenesis. Overall, the study emphasizes the significance of further research to fully understand the function of piRNAs in brain conditioning and their therapeutic potential.
Keywords: piRNA, PIWI, Neurological disorder, Brain conditioning, Small RNA, CNS
Abstract
This literature review focuses on the function of p-element-induced wimpy testis (PIWI)-interacting RNAs (piRNAs) in CNS disorders and their potential role in brain conditioning. While piRNAs have been widely investigated in germ cells, their role in brain function and neurological conditions remains unknown. This review gives an overview of piRNAs, their synthesis, and their interactions with PIWI proteins. It explores the potential function of piRNAs in gene regulation, chromatin remodeling, and interactions with other small RNAs. This review also investigates the role of piRNAs in diverse pathologies such as cancer, cardiovascular disease, reproductive system defects, and neurological diseases. In the setting of neurodegenerative disorders, deregulation of piRNA expression has been linked to Alzheimer's, Parkinson's, and Huntington's diseases, indicating their potential as therapeutic targets. The article continues to look at the role of piRNAs in the central nervous system, including their functions in inflammation, apoptosis, and neurogenesis. Overall, the study emphasizes the significance of further research to fully understand the function of piRNAs in brain conditioning and their therapeutic potential.
Keywords: piRNA, PIWI, Neurological disorder, Brain conditioning, Small RNA, CNS
Highlights
PiRNAs may play a significant role in the brain's response to stroke by regulating genes associated with neuroprotection and neuroinflammation. PiRNA expression is altered in response to stroke and may contribute to the brain's adaptive response to injury. In addition, piRNAs may be involved in regulating neural stem cell proliferation and differentiation, which is crucial for brain repair and recovery.
Introduction
The role of p-element-induced wimpy testis (PIWI)-interacting RNAs (piRNAs) in ischemic brain conditioning has not been extensively explored. This review aims to provide an in-depth understanding of the potential impact of piRNAs in this process and their potential as biomarkers akin to microRNAs (miRNAs).
PiRNAs are a subclass of small non-coding RNAs that function as regulatory molecules, like other regulatory RNA types such as micro RNAs (miRNAs) and small interfering RNAs (siRNAs). Unlike miRNAs, piRNAs are generally longer in length, typically ranging from 24 to 32 nucleotides, and exhibit limited sequence conservation. Furthermore, their biogenesis differs from miRNAs, as they are not reliant on the Dicer enzyme. PiRNAs often exhibit bias towards a 5' uridine and are frequently 2'-O-methylated at their 3' end (Horwich et al., 2007; Kirino and Mourelatos, 2007; Ohara et al., 2007). Notably, they represent the most diverse class of regulatory RNAs, with a vast number of unique piRNA sequences identified in various organisms. For instance, mice have over 68 million distinct piRNA sequences, Drosophila has over 41 million, and Caenorhabditis elegans has over 28,000 (Kim, 2019).
PiRNAs are commonly found in clusters throughout the genome and are particularly abundant in the germline. They originate from diverse sources, including transposons, protein-coding genes, and intergenic regions. PiRNAs interact with PIWI proteins, forming a complex that plays a crucial role in safeguarding genome integrity, particularly in the germline. However, piRNAs' specific involvement and potential implication in ischemic brain conditioning remain understudied.
This review seeks to fill a knowledge gap by researching the potential influence of piRNAs on the biological process and developing a thorough understanding of their pathophysiological role by investigating the changes associated with piRNA expression and their functional relevance. Furthermore, this review aims to evaluate the potential of piRNAs as biomarkers, similar to miRNAs, to enhance diagnostic and therapeutic strategies related to ischemic brain conditioning.
piRNA - History, nomenclature, and its physiological role
PIWI proteins are a family of RNA-binding proteins involved in piRNA-mediated gene silencing (Lee et al., 2012; Bortvin, 2013). PIWI (p-element induced wimpy testis) was discovered as an argonaute protein with a damaging impact on the testis in a PIWI KO model (Kim, 2019). PIWI proteins participate in germline preservation and transposon suppression (Houwing et al., 2007; Yakushev et al., 2016), as well as germline stem cell differentiation and growth. The PIWI protein family is related to PIWI-interacting RNAs (piRNAs), which are tiny RNAs 24-32 nucleotides long. They are RNA-binding proteins involved in gene regulation, particularly in the control of transposons (mobile genetic elements) and in the formation of germ cells (Thomson and Lin, 2009). Humans have four PIWI proteins: PIWIL1, PIWIL2, PIWIL3, and PIWIL4. In contrast, mice have three members in their PIWI protein family, MIWI (PIWIL1), MILI (PIWIL2), and MIWI2 (PIWIL4) (Grivna et al., 2006; Aravin et al., 2008) (Figure 1). MIWI knockout animals exhibit early spermatogenic abnormalities, whereas MILI and MIWI2 regulate transposon silencing in fetal gonocytes (Deng and Lin, 2002; Grivna et al., 2006).
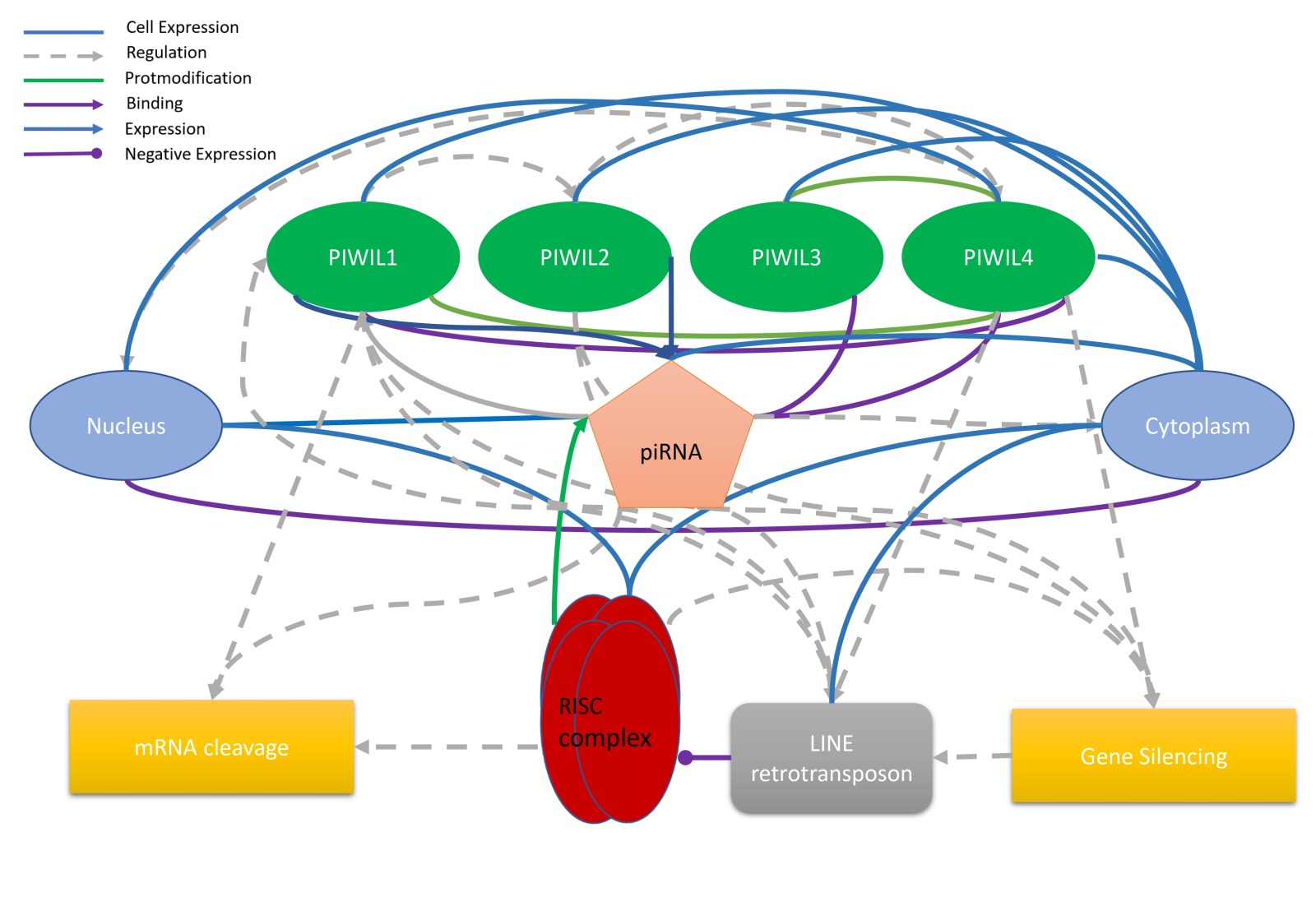
In a new window | Download PPT
Figure 1. Schematic representation of the piRNA pathway, supported by literature evidence. The figure illustrates the association between piRNAs and the piwi protein in the nucleus and cytoplasm, highlighting their role in mRNA cleavage and gene silencing. Additionally, the figure demonstrates the relationship between the RISC complex and the LINE retrotransposon.
PIWI proteins are the founding members of this protein family and are expressed in both germ and somatic cells (Thomson and Lin, 2009). They are known to be involved in maintaining genomic stability by suppressing transposable elements (TEs) and regulating gene expression. MILI (also known as PIWIL2) is a protein predominantly expressed in the germ cells of both males and females. It participates in the biogenesis of piRNAs (Kuramochi-Miyagawa et al., 2008; Siomi and Siomi, 2009). MIWI (also known as PIWIL1) is also predominantly expressed in male germ cells and plays a crucial role in spermatogenesis (Grivna et al., 2006). It aids in forming piRNA-induced silencing complexes, which help suppress the activity of TEs during sperm development (Deng and Lin, 2002; Grivna et al., 2006; Kuramochi-Miyagawa et al., 2008). All animal species have PIWI argonaute proteins and piRNAs, which play a crucial role in cell protection by blocking the initiation of transposons in the germline (Houwing et al., 2007; Sai lakshmi and Agrawal, 2008; Thomson and Lin, 2009) (Figure 1).
These non-coding RNAs, which can influence proteins involved in the neurogenetic process, like PIWI proteins, play a specific function in this process (Zhang et al., 2019). PIWI proteins and piRNAs are well expressed in brain cells, with the highest levels in hippocampal tissues (Perera et al., 2019). These findings suggest that neurogenesis may be connected to the function of neuronal piRNAs. However, mounting data point to the possibility that deregulation of the piRNA pathway causes genomic instability in neurons, which in turn promotes the emergence of several neurodegenerative diseases, including amyotrophic lateral sclerosis and Alzheimer's disease (Kim, 2019).
The significance of the role of piRNA in cellular homeostasis is anticipated to be more than 2.5 million interspersed repetitive elements, the majority of which are highly active transposons and retrotransposable elements. Many studies have shown that miRNA responds considerably more abundantly and faster than mRNA in the cerebral area during a focal ischemic stroke or an ischemic stroke (Lu et al., 2004; Kapadia et al., 2006; Jeyaseelan et al., 2008; Dharap et al., 2009, 2011). Considering there are more piRNAs than miRNAs, the probability of several piRNAs acting on the same site is likely, resulting in a cluster of piRNAs in the region, which is why many studies have failed to identify accession numbers, localization on chromosomes, or gene name(Sai lakshmi and Agrawal, 2008; Dharap et al., 2011).
One mechanism by which piRNAs may regulate gene expression is through their interaction with PIWI proteins (Watanabe and Lin, 2014). When piRNAs bind to PIWI proteins, they form a complex that recognizes and targets specific messenger RNAs (mRNAs) for degradation or translational repression (Rana, 2007; Filipowicz et al., 2008). In the brain, piRNA-mediated gene silencing may be important for regulating the expression of genes involved in synaptic plasticity and memory formation (Bagijn et al., 2012; Watanabe and Lin, 2014).
Another mechanism by which piRNAs may regulate gene expression in the brain is through their interaction with chromatin-modifying enzymes (Holoch and Moazed, 2015; Zhang et al., 2023). Studies have shown that piRNAs can recruit chromatin-modifying enzymes, such as histone methyltransferases and histone deacetylases, to specific genomic loci (Huang et al., 2013; Le Thomas et al., 2013). These enzymes can modify the structure of chromatin, making it accessible to transcription factors and other regulatory proteins. In the brain, piRNA-mediated chromatin remodeling may be important for regulating the expression of genes involved in synaptic plasticity and learning (Batista et al., 2008; Das et al., 2008; Huang et al., 2013; Le Thomas et al., 2013; Holoch and Moazed, 2015; Meseure et al., 2020; Su et al., 2020; Zoch et al., 2020; Zhang et al., 2023).
Finally, piRNAs may also regulate gene expression in the brain by interacting with other small RNA molecules, such as miRNAs (Du et al., 2016). Studies have shown that piRNAs and miRNAs can interact with each other and regulate each other's activity. In the brain, piRNA-miRNA interactions could be important for fine-tuning gene expression in response to environmental stimuli (Du et al., 2016).
piRNA in pathologies
Cancer
Numerous studies have reported the involvement of piRNAs in various cancers, including breast cancer, ovarian cancer, lung cancer, bladder cancer, and multiple myeloma (Zhang et al., 2013; Chu et al., 2015; Fu et al., 2015; Lee et al., 2016; Peng et al., 2016; Singh et al., 2018; Ai et al., 2019; Li et al., 2019). These studies suggest that piRNAs play a crucial role in cancer development and progression by regulating various biological processes such as cell proliferation, apoptosis, and invasion. For example, the first proof of a piRNA-mediated epigenetic mechanism capable of influencing cancer-related processes in the human breast is based on an investigation of piR-021285-responsive gene methylation (Fu et al., 2015). According to this study, the Akt/mammalian target of rapamycin (mTOR) pathway is influenced by piR-55490. Interestingly, Law et al. (2013) observed that silencing piR-Hep1 led to the inhibition of the Akt pathway in liver cancer cells, indicating that the Akt pathway may be a frequent target of piRNA action. Unfortunately, the authors did not investigate the molecular mechanism of piR-Hep1's influence Akt/mTOR activation. However, it supports the idea that the degradation of mTOR, a key component of this pathway, is crucial for piRNA's biological role in cancer cells (Peng et al., 2016, 2021). These findings suggest that piRNAs could serve as potential therapeutic targets for cancer treatment.
Cardiovascular disease
The function of piRNAs has been extensively studied in cardiac progenitor cells such as cardiofibroblasts, cardiosphere-derived cells, and cardiospheres (Vella et al., 2016; Wu et al., 2020). Increased expression of piRNAs such as DQ591926, DQ593270, and DQ593595 were discovered to target transposons in the human genome, namely long interspersed element (LINE) retrotransposons, with LINE-1 being the most targeted (Vella et al., 2016). Via the AKT signaling pathway, these piRNAs also increased cell viability in the cardiac system (Rajan et al., 2014). Several piRNAs were reported to be downregulated in heart failure patients in another study, with hsa-piR-020009 and hsa-piR-006426 being the top two (Yang et al., 2018). Except in troponin-I-negative myocardial infarction patients, piR-2106027 levels in myocardial infarction patients were considerably higher (Rajan et al., 2016).
Reproductive system
In underdeveloped countries, one out of every four couples suffers from infertility, and patients who suffer from infertility undergo psychological stress, which may lead to mental health issues (Vander Borght and Wyns, 2018). Medical disorders primarily cause female infertility, but male infertility may be caused by testicular and post-testicular deficiencies (Vander Borght and Wyns, 2018). Abnormal piRNA expression in testicular cells may cause spermatogenic failure. Downregulation of five piRNAs (DQ589977, DQ591415, DQ598918, DQ601291, and DQ601609) has been linked to spermatogenic failure, and piRNAs have been discovered as effective tools for infertility diagnosis as well as prospective treatment targets for novel drugs (Heyn et al., 2012). It is hypothesized that determining cell and stage-specific piRNA expression patterns during mammalian spermatogenesis may be advantageous (Cao et al., 2018; Kamaliyan et al., 2018; Kumar et al., 2019).
Neurodegenerative diseases
PiRNAs interact with PIWI proteins and play a critical role in germ cell development and maintenance. Recent studies have shown that piRNAs are also expressed in the brain, suggesting a potential role in neurodevelopment and neurodegenerative diseases. Chavda et al. (2022) reviewed the role of piRNAs in neurodevelopment and neurodegenerative disorders. They reported that piRNAs regulate gene expression, chromatin structure, and epigenetic modifications and may play a role in maintaining neuronal homeostasis (Figure 2). Changes in piRNA expression have shown associations with various neurodegenerative conditions like Alzheimer's disease, Parkinson's disease, Huntington's diseases, and amyotrophic lateral sclerosis.

In a new window | Download PPT
Figure 2. Illustratration of the role of piRNA in a different molecular pathway.
Similarly, Huang and Wong (2021) explored the role of piRNAs in neurodegenerative diseases. They highlighted that piRNAs are involved in RNA silencing pathways and can regulate gene expression at the post-transcriptional level. In order to comprehend the role of piRNAs in neuronal activity and transcriptional regulation, it is necessary to assess their roles and targets as they are extensively expressed in the brain and dysregulated in neurological disorders..
In another study, the authors reviewed the role of piRNAs in various neuronal disorders, including Alzheimer's disease, Huntington's disease, and schizophrenia (Wakisaka and Imai, 2019). They reported that piRNAs may regulate the expression of disease-associated genes and play a role in disease pathogenesis. The authors suggested that targeting piRNAs may have therapeutic potential for these diseases.
Finally, (Kuo et al., 2021) reviewed the role of non-coding RNAs, including piRNAs, in Parkinson's disease (PD). They reported that dysregulation of non-coding RNAs has been implicated in PD pathogenesis and that piRNAs may play a role in regulating mitochondrial function and oxidative stress, both of which are implicated in PD. The authors suggested that piRNAs may be used as biomarkers for PD diagnosis and prognosis.
In conclusion, piRNAs are a class of small non-coding RNAs that play a crucial role in maintaining neuronal homeostasis and have been implicated in various neurodegenerative diseases. The dysregulation of piRNA expression has been suggested as a potential therapeutic target for these diseases. Further research is needed to fully understand the role of piRNAs in neurodegenerative diseases and their potential as therapeutic targets.
Neurological conditions such as stroke and neurodegenerative diseases may also have a substantial impact on a person's quality of life. Although the etiology of different diseases differs, they share certain similarities in their impact on the brain. Both neurodegenerative diseases and stroke can induce brain damage, resulting in cognitive and physical deficits. Furthermore, some neurodegenerative disorders, such as Alzheimer's and Parkinson's, can increase the risk of stroke, and having a stroke may result in cognitive impairment comparable to that found in neurodegenerative diseases. While they are different ailments, there is a relationship between neurodegenerative diseases and stroke that highlights the significance of initial detection and therapy for these problems to reduce their consequences on patients.
PiRNAs function in the central nervous system
Inflammation
Research has shown that piRNA plays a crucial role in maintaining the integrity of the blood-brain barrier (BBB) and regulating inflammation in the central nervous system (CNS) (Liu et al., 2021). In particular, studies have identified a specific piRNA, DQ593109, and its binding protein, PIWIL1, as critical regulators of BBB permeability via the maternally expressed 3 /miR-330-5p/Runt-related transcription factor 3 axis (Shen et al., 2018). Furthermore, PIWI has been identified as a potential molecular bridge between the BBB and neuropathological conditions, suggesting that targeting piRNA and PIWI may offer a novel therapeutic approach for BBB dysfunction and related disorders (Roy et al., 2020) (Figure 2). These findings underscore the importance of non-coding RNA in BBB regulation and provide new insights into the mechanisms underlying neuroinflammation (Shen et al., 2018; Roy et al., 2020; Liu et al., 2021).
Apoptosis
PiRNAs may also play a role in regulating apoptosis or programmed cell death, which is a common feature of stroke. Studies have shown that some piRNAs can target and regulate gene expression in apoptosis.
Neurogenesis
The piRNA pathway is critical for sustaining adult neurogenesis in the CNS, as piRNAs are expressed in neural stem cells and are necessary for maintaining a functional neural stem cell pool (Gasperini et al., 2023). Depletion of piRNAs led to an impairment in neural stem cell proliferation and differentiation, highlighting piRNAs' essential role in maintaining adult neurogenesis (Altman, 1962; Doetsch et al., 1999). Moreover, piRNAs play a crucial role in reducing cellular senescence and promoting cellular homeostasis by regulating genes involved in protein synthesis (Gasperini et al., 2023). This is significant because cellular senescence can impair neural stem cell function, ultimately leading to age-related neurodegenerative diseases (Encinas et al., 2011; Clarke et al., 2018). Furthermore, piRNAs protect neural stem cells against oxidative stress-induced DNA damage by activating the phosphoinositide-3-kinase\AKT signaling pathway (Adusumilli et al., 2021; Gasperini et al., 2023). This finding is crucial because oxidative stress and DNA damage can impair neural stem cell function, leading to a decline in neurogenesis and an increased risk of neurodegenerative diseases.
In summary, piRNAs play a role in maintaining adult neurogenesis in the CNS by regulating protein synthesis, cellular senescence, and neuroprotection. Understanding how piRNAs regulate neurogenesis could lead to new therapies to combat age-related neurodegenerative diseases (Gasperini et al., 2023).
Angiogenesis
PiRNAs may also play a role in regulating angiogenesis or the formation of new blood vessels, which is a key process in stroke recovery. Studies have shown that some piRNAs regulate the expression of genes that promote angiogenesis (Figure 2). Numerous studies have shown that piRNAs can play a crucial role in regulating the process of angiogenesis (Pi et al., 2023). In one such study, the primary objective was to investigate the potential of naringin in promoting angiogenesis and bone regeneration, both in vitro and in vivo, and to explore the underlying mechanisms involved. Understanding whether naringin may affect angiogenesis by modifying piRNAs and associated target genes is essential. In support of this research, a previous study demonstrated that inhibiting piRNAs can lower vascular endothelial growth factor expression in endothelial cells, which in turn lowers the quantity of neovascular arteries (Li et al., 2019). Furthermore, piRNA-823 expression was elevated in exosomes from multiple myeloma cells when co-cultured with vascular endothelial cells, resulting in increased expression of vascular endothelial growth factor and interleukin-6 in the endothelial cells (Yan et al., 2014). Hence, piRNAs can mediate angiogenesis through various pathways, and this study aims to investigate the role of naringin in regulating angiogenesis and its associated molecular mechanisms.
Gene regulation
DNA methyltransferase 3 (DNMT3) is a DNA methyltransferase involved in epigenetic gene expression regulation (Rayford et al., 2021; Zhan et al., 2023). DNA methylation can regulate gene expression by adding a methyl group to the DNA molecule, which can change the activity of DNA-binding proteins and alter gene expression. Evidence suggests that piRNAs may regulate stroke by interacting with the DNMT3 gene (Rayford et al., 2021; Zhan et al., 2023). One research paper found that hypoxic postconditioning reduced the expression of Piwil2 and DNMT3 in a rat model of cerebral ischemia, leading to decreased DNA methylation at the cyclic AMP response element-binding 2 (CREB2) promoter, a gene involved in cerebral ischemic injury (Zhan et al., 2023). The authors suggest that piRNA-mediated regulation of DNMT3 may be a key mechanism by which hypoxic postconditioning protects against cerebral ischemic injury. Rayford et al. (2021) note that piRNAs can regulate DNMT3 expression by interacting with the gene's 3' untranslated region (UTR). Dysregulation of this process may contribute to the development and progression of various diseases, including stroke.
Regulation of TEs
TEs are mobile genetic elements that can disrupt genomic stability and gene expression. In the germline, piRNAs have been shown to regulate TEs and prevent their harmful effects. Recent studies suggest piRNAs may have a similar role in the central nervous system (CNS) (Figure 1 and 2). In the mouse brain, piRNAs regulate TEs in neural progenitor cells and help maintain genomic stability during neurodevelopment (Ghosheh et al., 2016). Dysregulation of the piRNA pathway can lead to TE activation, which can cause genomic instability and diseases (Zhang and Wong, 2022). The piRNA-PIWI complex guides PIWI proteins to target transposons through sequence complementarity, triggering various mechanisms to suppress TE activity and maintain genome integrity, such as transcriptional and post-transcriptional silencing and chromatin remodeling (Vourekas et al., 2012). PiRNA biogenesis is triggered by trans-generationally inherited piRNAs, which change the chromatin of piRNA clusters and induce precursor processing (Vourekas et al., 2012). Peptide nucleic acid-mediated gene silencing has also been used to investigate the role of piRNAs in regulating gene expression in vitro and in vivo (Aravin et al., 2008). The link between individual TEs and de novo DNA methylation in mice highlights the importance of piRNA regulation in maintaining genomic integrity (Aravin et al., 2008). Overall, these studies suggest that piRNAs have diverse roles in the CNS. Further research will be needed to fully understand the functions of piRNAs in the CNS and how they may contribute to neurological disorders.
Brain conditioning and neurogenesis
Brain conditioning is a phenomenon where brief, non-lethal periods of neural activity can confer protection against subsequent neural injury. This phenomenon has been observed in various contexts, including ischemic stroke, traumatic brain injury, and neurodegenerative diseases. Recent studies have suggested that piRNAs may play a role in the neuroprotective effects of brain conditioning.
Stroke is a leading cause of death and disability worldwide, and current treatments for stroke have limited effectiveness. There is a growing interest in exploring novel therapeutic targets for stroke, and recent studies suggest that the piRNA pathway may be a promising target. PiRNAs are small, non-coding RNAs implicated in various biological processes, including neurogenesis and cellular senescence. In this section, we will discuss and review how the piRNA pathway may play a role in brain conditioning and neurogenesis in stroke based on research papers published in relation to neurogenesis.
The study by (Gasperini et al., 2023) investigated the role of the piRNA pathway in sustaining neurogenesis and preventing cellular senescence in the postnatal hippocampus. The researchers focused on a specific piRNA, Piwil2 (also known as Mili), which is expressed in neural stem cells and progenitor cells (Ahlenius et al., 2009; Walker and Kempermann, 2014; Pons-Espinal et al., 2017). They found that Piwil2-deficient mice exhibited reduced neurogenesis and increased cellular senescence in the hippocampus (Gasperini et al., 2023). Furthermore, Piwil2 overexpression increased the proliferation and differentiation of neural stem cells while reducing cellular senescence. These findings suggest that Piwil2 plays a critical role in maintaining the proper balance between neurogenesis and senescence in the hippocampus (Gasperini et al., 2023).
The relevance of the piRNA pathway in maintaining adult neurogenesis was further investigated by Gasperini et al. (2023). Based on the findings, the piRNA pathway is critical in lowering protein production and cellular senescence in neural stem cells, increasing neurogenesis (Gasperini et al., 2023). The piRNA pathway regulates TEs in the genome and maintains genomic stability. According to the study, the loss of piRNA in neural stem cells causes increased protein synthesis and cellular senescence, which are detrimental to neurogenesis. According to the researchers, targeting the piRNA pathway might be an intriguing approach to brain conditioning and treating neurological disorders (Gasperini et al., 2023).
In addition to regulating neurogenesis, piRNAs have been implicated in regulating gene expression in the nervous system. Iyenger et al. (2014) reviewed the role of non-coding RNAs, including piRNAs, in regulating neuronal development and function. They highlighted several studies showing that piRNAs can regulate gene expression in neurogenesis, synaptic plasticity, and neuronal differentiation (Lee et al., 2011; Rajasethupathy et al., 2012). These findings suggest that piRNAs may play a broader role in shaping the development and function of the nervous system beyond their role in regulating neurogenesis (Iyengar et al., 2014).
The studies above suggest that the piRNA pathway may be involved in brain conditioning and neurogenesis in stroke. Wakisaka and Imai (2019) reviewed the emergence of piRNA research in various neuronal disorders and highlighted several studies that implicate the piRNA pathway in regulating neuronal survival and plasticity in response to injury. They also discussed the potential therapeutic applications of targeting the piRNA pathway in these disorders, including stroke. Kim (2019) reviewed the role of PIWI proteins and piRNAs in the nervous system and discussed their potential as therapeutic targets for neurodegenerative diseases and brain injury. These studies suggest that the piRNA pathway may be a promising therapeutic target for promoting neurogenesis and enhancing recovery after stroke.
To summarize, the piRNA pathway is a new area of study with intriguing implications for stroke and other neurological conditions. According to the findings presented in this section, piRNAs, notably Piwil2, have an important function in controlling neurogenesis and cellular senescence in the brain. Targeting the piRNA pathway may be an effective approach for elevating neurogenesis and improving stroke recovery, and further study in this area is warranted.
piRNA and the other classes of non-coding RNA
piRNAs and miRNAs
Studies on the interaction between piRNAs and miRNAs have revealed that small non-coding RNA molecules play significant roles in gene expression regulation in various organisms (Du et al., 2016). In mice, piRNAs can negatively regulate miRNA expression by promoting the degradation of miRNA precursors, while miRNAs can positively regulate piRNA expression by targeting and cleaving piRNA precursor transcripts (Du et al., 2016). In humans, piRNAs interact with the AGO2 protein, which is also involved in miRNA-mediated gene silencing. These findings indicate that there might be complex interactions between piRNAs and miRNAs in gene regulation (Figure 3).
Despite their similar functions and size, miRNAs and piRNAs are distinct RNA classes with different biogenesis pathways and functions. miRNAs are produced by the Dicer enzyme from double-stranded RNA precursors, while piRNAs are produced by the PIWI proteins from single-stranded RNA precursors. miRNAs are expressed in various tissues and cell types, while piRNAs are primarily expressed in germline cells (Thomson and Lin, 2009). miRNAs generally target mRNAs with complementary sequences in their 3' untranslated regions, while piRNAs primarily target TEs and repeat sequences in the genome (Seto et al., 2007) (Figure 1). While miRNAs primarily function as negative gene expression regulators, piRNAs maintain genome stability by silencing TEs and protecting the germline genome from transposon-mediated mutations (Le Thomas et al., 2014; Zhang et al., 2019) (Figure 4). Nonetheless, both RNA molecules regulate gene expression and have been linked to various biological processes such as development, differentiation, and disease (Suh and Blelloch, 2011).
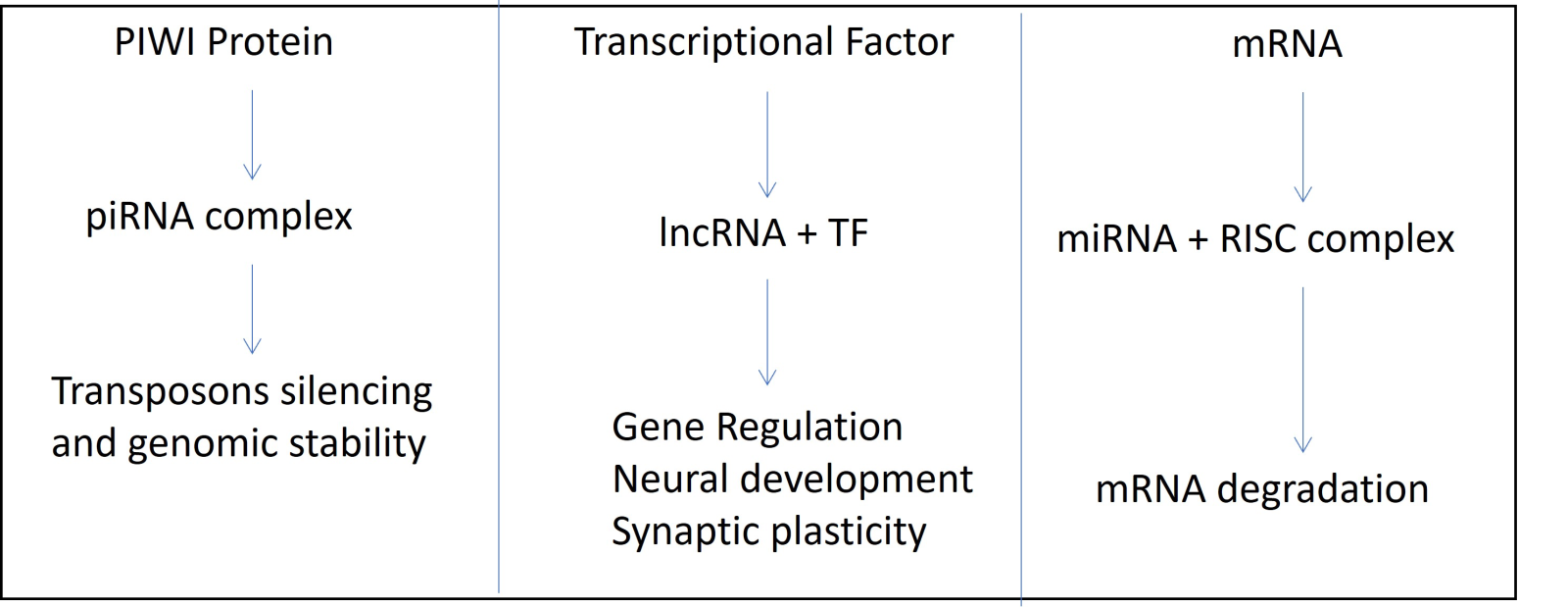
In a new window | Download PPT
Figure 4. Flowchart depicting three possible mechanisms of gene expression regulation: (A) PIWI Protein; (B) Transcriptional factors; (C) mRNA and RISC complex.
piRNAs and long non-coding RNAs
The relationship between piRNA and long non-coding RNA (lncRNA) has been demonstrated in various ways (Wang and Lin, 2021). During the pachytene stage, some piRNA clusters arise from testis-specific lncRNAs, indicating that lncRNAs can act as piRNA precursors (Li et al., 2013; Gainetdinov et al., 2017). The presence of transposon families like human endogenous retrovirus subfamily H in lncRNAs of human embryonic stem cells and induced pluripotent stem cells correlates with high expression levels of host lncRNAs, suggesting that human endogenous retrovirus subfamily H sequences positively regulate the expression of lncRNAs in a cell-type-specific manner. However, the regulatory mechanism is not yet known (Kelley and Rinn, 2012). In Drosophila ovarian somatic sheet and ovarian somatic cells, transposon insertions around lncRNAs stimulate the expression of corresponding lncRNAs in a PIWI-dependent manner, suggesting the possible involvement of piRNAs in transcriptional regulation of lncRNA expression (Sytnikova et al., 2014). Additionally, some lncRNAs produce piRNAs that promote gene expression at the transcriptional level. In the human breast cancer cell line MCF7, growth arrest-specific-5-derived piRNAs associated with PIWI proteins facilitate the transcription of tumor necrosis factor-related apoptosis-inducing ligand mRNA by recruiting the epigenetic modifier WD repeat domain 5 and the complex proteins associated with Set1 complex to the tumor necrosis factor-related apoptosis-inducing ligand promoter (He et al., 2015). Tetrahymena piRNAs bind to Tetrahymena PIWI protein Twi8p and its target lncRNAs, leading to decreased levels of the targeted lncRNAs (Farley and Collins, 2017). These roles are analogous to those found in mammalian systems. Although the exact mechanisms of this interaction are not yet fully understood, it is believed that piRNAs and lncRNAs may regulate each other's expression or stability. These findings highlight the intricate relationship between piRNAs and lncRNAs and their roles in gene regulation.
piRNAs and small nucleolar RNAs (snoRNA)
Research by Zhong et al. (2015) observed a snoRNA-derived piRNA interacting with human interleukin-4 pre-mRNA, leading to its degradation in nuclear exosomes. The study reveals a novel mechanism of gene regulation and suggests that non-coding RNAs, such as snoRNAs and piRNAs, may have more complex roles in gene expression than previously known. The findings also provide insight into the potential crosstalk between different types of non-coding RNAs in gene regulation (Zhong et al., 2015).
Limitations
While piRNAs have beneficial effects in various biological processes, including gene regulation and transposon silencing, there are some limitations to their use:
Lack of functional characterization: Despite the growing interest in piRNAs, their functional characterization remains incomplete. Many identified piRNAs have yet to be assigned a specific function, and it is unclear whether they have any biological relevance.
Challenges in piRNA detection: Detecting piRNAs can be challenging, as they are present in low abundance and can be difficult to distinguish from other small RNAs, such as microRNAs. Furthermore, piRNAs can have significant sequence variation, making it difficult to design specific probes or primers for detection.
Limited understanding of piRNA biogenesis: Although progress has been made in understanding the biogenesis of piRNAs, many aspects of their biogenesis remain unclear. For example, the exact mechanisms by which piRNAs are processed and loaded onto PIWI proteins are still not fully understood.
Limited knowledge of piRNA targets: While some piRNA targets have been identified, many of the biological targets of piRNAs remain unknown. This makes it difficult to fully understand the regulatory mechanisms by which piRNAs operate.
Species-specificity: piRNAs are highly variable across different species, and the piRNA repertoire of one species may not be directly translatable to another species. This can limit the utility of piRNA research across different species.
Overall, while piRNAs have beneficial effects in various biological contexts, there are still many limitations and challenges associated with their use that must be addressed to fully understand their potential for research and therapeutic applications.
Conclusion
Research suggests that piRNAs may significantly affect the brain's response to stroke by regulating genes associated with neuroprotection and neuroinflammation (Shen et al., 2018; Roy et al., 2020; Liu et al., 2021). Studies have also shown that piRNA expression is altered in response to stroke and may contribute to the brain's adaptive response to injury (Dharap et al., 2011). In addition, piRNAs may be involved in regulating neural stem cell proliferation and differentiation, which is crucial for brain repair and recovery (Gasperini et al., 2023). While further research is needed to fully understand the role of piRNAs in brain conditioning following stroke, these findings suggest that targeting piRNAs could be a promising strategy for developing new therapies for stroke. By regulating critical processes involved in the brain's response to injury, piRNAs could potentially improve functional recovery and contribute to the repair and recovery of the brain following a stroke.
Conflict of interest
The authors declare that they have no conflicts of interest.GP is on the Editorial Board for Conditioning Medicine. He has not participated at any level in the editorial review of this manuscript.
Acknowledgments
This work was supported by grant CN00000041 “National Center for Gene Therapy and Drugs based on RNA Technology” (concession number 1035of 17 June 2022-PNRR MUR – M4C2 – Investment 1.4 Call “National Centers”, financed by EU- NextGenerationEU), code project (CUP) E63C22000940007 to GP and OC.
RMP: Conceptualization and Writing–original draft. OC: Editing GP: Funding acquisition, Conceptualization, Supervision, Validation, Writing–review and editing.
References
Rohan Mahesh Patil1
1University of Naples “Federico II”, School of Medicine and Surgery, Neuroscience, Napoli, Italy.
Ornella Cuomo1
1University of Naples “Federico II”, School of Medicine and Surgery, Neuroscience, Napoli, Italy.
Giuseppe Pignataro1
1University of Naples “Federico II”, School of Medicine and Surgery, Neuroscience, Napoli, Italy.
Corresponding author:
Giuseppe Pignataro
Email: gpignata@unina.it

In a new window | Download PPT
Figure 1. Schematic representation of the piRNA pathway, supported by literature evidence. The figure illustrates the association between piRNAs and the piwi protein in the nucleus and cytoplasm, highlighting their role in mRNA cleavage and gene silencing. Additionally, the figure demonstrates the relationship between the RISC complex and the LINE retrotransposon.

In a new window | Download PPT
Figure 2. Illustratration of the role of piRNA in a different molecular pathway.

In a new window | Download PPT
Figure 4. Flowchart depicting three possible mechanisms of gene expression regulation: (A) PIWI Protein; (B) Transcriptional factors; (C) mRNA and RISC complex.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5682 | 46 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA
