Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Current therapies targeting inflammation following ischemia-reperfusion injury in the diabetic heart
Time:2024-03-08
Number:4327
Linh Chi Dam1,2, William Boisvert3, Sauri Hernandez-Resendiz1,2
Author Affiliations


- 1Cardiovascular and Metabolic Disorder Program, Duke-NUS Medical School, Singapore.
- 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
- 3Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA.
Conditioning Medicine 2023. 6(4): 120-130.
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often follows are major causes of death and disability worldwide. The detrimental effects of AMI on the myocardium often result from the effects of acute ischemia and reperfusion injury (IRI). Moreover, patients with type 2 diabetes mellitus (DM) are more susceptible to acute myocardial IRI and post-infarction adverse cardiac remodeling. Following an AMI, DM patients also have worse outcomes, including progression to HF and death. Thus, there is an urgent need to develop cardioprotection strategies to reduce acute myocardial IRI and prevent adverse cardiac remodeling post-AMI, which are effective in the diabetic heart. The inflammatory response plays a crucial role in both IRI and DM environments, influencing infarct size, adverse cardiac remodeling, and the healing phase post-MI. Further, it constitutes a chronic low-grade tissue inflammation in DM. In this review, we will discuss inflammation in the context of both DM and myocardial IRI and highlight potential therapies targeting inflammation in preclinical and clinical studies that have the potential to improve AMI outcomes in diabetic patients.
Keywords: Myocardial ischemia/reperfusion injury, Diabetes mellitus, Murine diabetic models, Immunotherapy
Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often follows are major causes of death and disability worldwide. The detrimental effects of AMI on the myocardium often result from the effects of acute ischemia and reperfusion injury (IRI). Moreover, patients with type 2 diabetes mellitus (DM) are more susceptible to acute myocardial IRI and post-infarction adverse cardiac remodeling. Following an AMI, DM patients also have worse outcomes, including progression to HF and death. Thus, there is an urgent need to develop cardioprotection strategies to reduce acute myocardial IRI and prevent adverse cardiac remodeling post-AMI, which are effective in the diabetic heart. The inflammatory response plays a crucial role in both IRI and DM environments, influencing infarct size, adverse cardiac remodeling, and the healing phase post-MI. Further, it constitutes a chronic low-grade tissue inflammation in DM. In this review, we will discuss inflammation in the context of both DM and myocardial IRI and highlight potential therapies targeting inflammation in preclinical and clinical studies that have the potential to improve AMI outcomes in diabetic patients.
Keywords: Myocardial ischemia/reperfusion injury, Diabetes mellitus, Murine diabetic models, Immunotherapy
Highlights
This review manuscript discusses the inflammatory responses of type 2 diabetic hearts in response to acute myocardial ischemia-reperfusion injury. The review then focuses on promising therapies directed at these inflammation targets in recent preclinical and clinical studies, which can progress these molecular insights toward clinically effective for diabetic patients with acute myocardial infarction. This altogether summarizes the potential new drug targets and therapeutic approaches in conditioning medicine for cardiovascular diseases.
Introduction
Acute myocardial infarction (AMI) and the heart failure (HF) that can follow are among the leading causes of death and disability worldwide. The detrimental effects of AMI on the myocardium result from the combined effects of acute ischemia and reperfusion injury (IRI) (Heusch, 2020). The treatment of choice for AMI is timely reperfusion using percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG), which is often decided based on patients’ current clinical stage, clinical outcome, sex, and age (Verma et al., 2002) However paradoxically, the process of restoring blood flow to the ischemic myocardium can itself induce further myocardial injury and cardiomyocyte death – a phenomenon termed lethal reperfusion injury that can itself contribute up to 50% to the final infarct size. (Yellon and Hausenloy, 2007; Hausenloy et al., 2016; Heusch et al., 2020). Despite success in animal studies, the translation of different cardioprotective therapies to reduce myocardial IRI to clinical practice has been limited (Davidson et al., 2019). This lack of successful translation to the clinical setting may be explained by differences between pre-clinical models and the clinical status of the patients, including age, comorbidities, and co-medications. Most cardioprotective therapies have been investigated using healthy young male animals in preclinical models. Patients are often older or have other comorbidities, which include type 2 diabetes mellitus (T2DM), that complicate the response to different cardioprotective therapies (Ferdinandy et al., 2023).
T2DM patients are more prone to acute myocardial IRI and adverse left ventricle (LV) remodeling post-AMI, putting them at great risk of developing HF (Whittington et al., 2012; Ferdinandy et al., 2023). T2DM patients are also less responsive to cardioprotective strategies (Kleinbongard et al., 2020).
Following AMI, T2DM patients also have worse mortality outcomes than patients without diabetes (Donahoe et al., 2007; Jimenez-Quevedo et al., 2019). However, the underlying mechanisms remain incompletely understood. Therefore, there is an urgent need to develop cardioprotective strategies to reduce acute myocardial IRI and prevent adverse LV remodeling post-AMI, which are effective in the diabetic heart.
Inflammation is an important contributor to acute myocardial IRI and the adverse LV remodeling that occurs post-AMI. However, inflammation is not limited to the heart but is altered in various conditions, including comorbidities such as T2DM. It is well established that chronic, low-grade inflammation drives cardiovascular disease in the diabetic heart, and the process of inflammation provides the pathological link between diabetes and acute myocardial IRI. Nonetheless, there are still currently no effective pharmacological interventions to reduce inflammation in diabetic myocardial IRI. In this review, we discuss inflammation in the context of both T2DM and myocardial IRI and highlight potential therapies for targeting inflammation in preclinical and clinical studies that have the potential to improve AMI outcomes in diabetic patients.
Inflammation in acute myocardial ischemia/reperfusion injury in diabetic heart
Inflammation plays a key role in mediating the detrimental effects of acute myocardial IRI and subsequent adverse LV remodeling (Ong et al., 2018; Algoet et al., 2022). Inflammation is initiated during myocardial ischemia and becomes the leading player in tissue damage in the adverse remodeling process (Hausenloy and Yellon, 2013; Hausenloy et al., 2016). Following an AMI, the hostile environment of the infarcted area triggers sterile inflammation, including the release of danger-associated molecular patterns (DAMPs) from injured cardiac parenchymal cells or damaged extracellular matrix components (Algoet et al., 2022). These DAMPs will bind to pattern recognition receptors (PRRs) on parenchymal cells, including cardiac fibroblasts, resident macrophages, and infiltrating leukocytes, and activate the mitogen-activated protein kinases (MAPKs) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) pathways, as well as the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome (Algoet et al., 2022).
Inflammasomes are multi-protein complexes that trigger inflammatory responses upon recognizing pathogens or endogenous danger signals. The NLRP3 inflammasome complex contains a sensor molecule (NLRP3), an adaptor (apoptosis-associated speck-like protein containing a CARD [ASC], also known as PYCARD), and an effector (caspase-1) (Swanson et al., 2019). When the NLRP3 sensor molecule senses a pathogen-associated molecular pattern (PAMP) or host-derived DAMPs, it will recruit the adaptor protein ASC. ASC self-associates into a helical fibrillary assembly, forming an ASC speck or pyroptosome (Mangan et al., 2018). ASC speck subsequently recruits and activates the effector caspase-1, resulting in proteolytic cleavage and secretion of potent pro-inflammatory cytokines interleukin (IL)-1β, IL-18, and inflammatory lytic cell death called pyroptosis. Pyroptosis further releases inflammatory lipid mediators and ASC specks, propagating inflammation by activating surrounding macrophages. The activation of NLRP3 inflammasome and NF-kB pathways thus drives the expression of multiple pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), IL-6, IL-1β, and IL-18 (Liu et al., 2017; Swanson et al., 2019). The activation of NLRP3 inflammasome further recruits neutrophils and monocytes from the bone marrow and the spleen to the infarcted heart and amplifies the inflammatory response (Swirski et al., 2009). In addition to bone marrow monocytes, Ly-6Chigh (Gr1hiCCR2+CX3CR1lo) and Ly-6Clow (Gr1loCCR2-CX3CR1hi) monocytes are also mobilized from the spleen post-MI and contribute significantly to digesting damaged tissues and wound healing (Nahrendorf et al., 2007; Swirski et al., 2009). These infiltrating leukocytes and cardiac resident macrophages are primarily responsible for the inflammatory response. To demonstrate the crucial role of NLRP3 inflammasome activation in IRI, a mouse model of AMI treated with NLRP3 inflammasome inhibitor, ASC knockout mice, and mice with genetic deletion of caspase-1 have been shown to provide cardioprotection following AMI (Frantz et al., 2003; Kawaguchi et al., 2011; Marchetti et al., 2014).
In addition, mitochondria also generate large quantities of reactive oxygen species (ROS) in response to IRI. ROS play an important role in initiating and maintaining inflammatory reactions as they act as chemoattractants to recruit circulating leukocytes (Hausenloy and Yellon, 2013; Hausenloy et al., 2016). Furthermore, restoring blood flow during reperfusion may augment tissue damage through additional mitochondria and mitochondrial damage associated ROS generation and activation of the complement pathways. In the first few minutes of myocardial reperfusion, ROS is generated in a burst by dysfunctional mitochondria (Hausenloy and Yellon, 2013; Heusch, 2020). The details of the inflammatory response following AMI have been carefully explained in the following reviews (Franogiannis, 2014; Ong et al., 2018; Algoet et al., 2022).
Macrophages are crucial cell types in IRI, where cardiac monocytes and macrophage numbers expand rapidly in the days following AMI. The classical M1 macrophage phenotype is described to be pro-inflammatory, while M2 macrophage is associated with tissue repair and wound healing (Algoet et al., 2022). However, more recent data demonstrate the remarkable heterogeneity and plasticity in macrophage development, phenotype, and function following AMI, suggesting a network of diverse macrophage behaviors driven by a complex network of stimuli instead of just the traditional M1-M2 paradigm (Peet et al., 2020). Single-cell RNA sequencing (scRNA-seq) studies also highlighted that macrophage populations may have different roles depending on their location in the infarcted heart following AMI (infarct zone, border zone, and remote zone). In addition, there also appears to be significant temporal variation in macrophage phenotype from pro-inflammatory to reparative to resident-like following AMI (Mouton et al., 2018). The anti-inflammatory reparative phase is driven by the activation of specific endogenous inhibitory pathways that suppress inflammation and dynamic changes in the roles of infiltrating leukocytes (Frangogiannis, 2014; Algoet et al., 2022). When this transition is dysregulated, such as in the chronic low-grade inflammation state, adverse LV remodeling and heart failure can occur.
In T2DM patients, evidence suggests a chronic low-grade tissue inflammation state as a key feature, which consists of the recruitment, accumulation, and activation of pro-inflammatory macrophages in metabolic tissues, and other types of immune cells in these inflammatory processes (Rohm et al., 2016; Tsalamandris et al., 2019). The chronic inflammation results from long-term innate immune activation, with inflammatory cytokines such as IL-1β, IL-6, and TNF-α accumulated in tissues (adipose tissues, pancreas, and suggestion of others, such as liver and skeletal muscles) and in circulation. This might, in part, explain the increased susceptibility and worse outcomes of diabetic patients following AMI. However, there is currently a lack of studies delineating the molecular changes in inflammation and the immune system following acute myocardial IRI in the diabetic heart due to the challenges in spatial and temporal variations mentioned above and the complexity of inflammation.
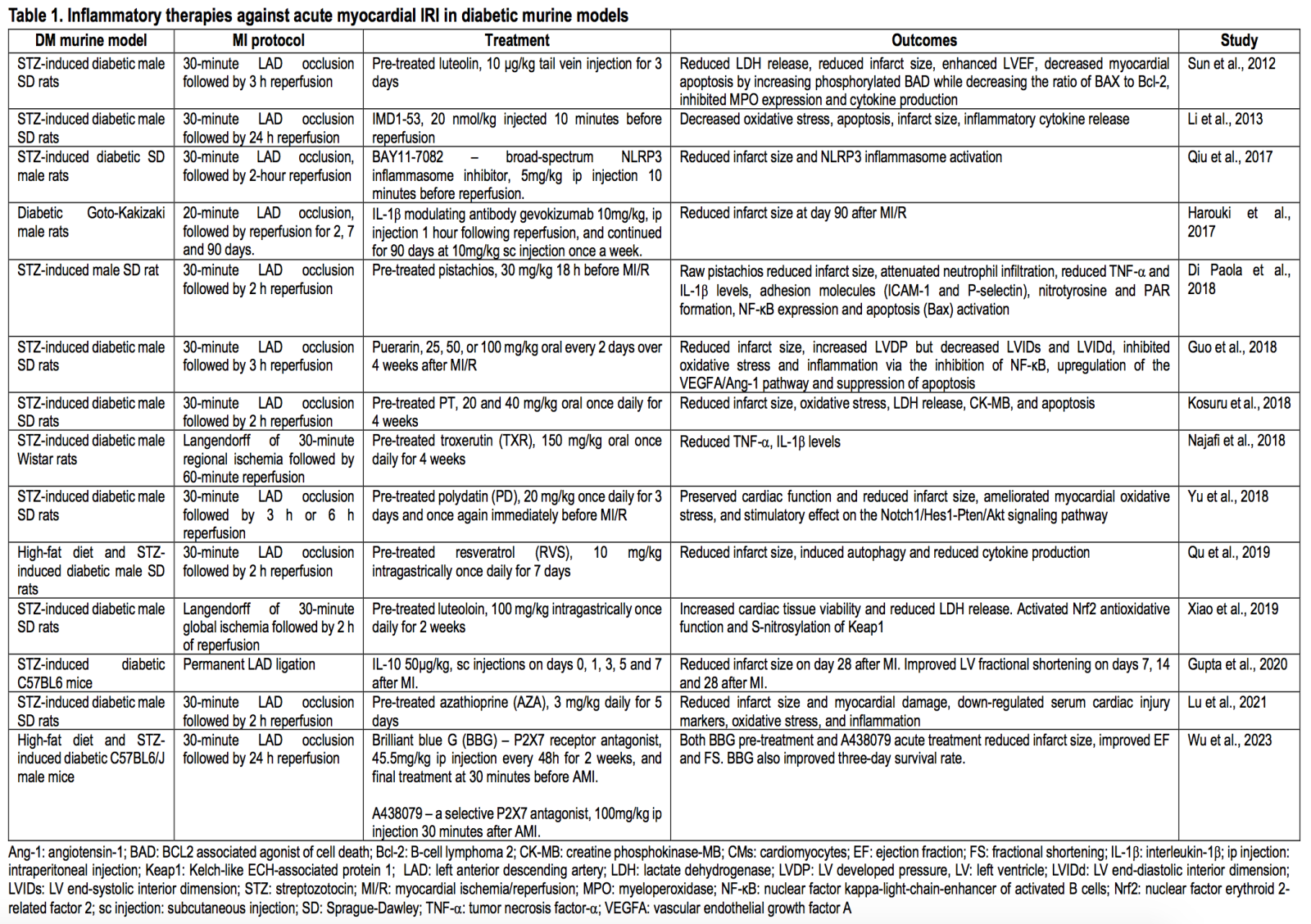
Targeting inflammation in the diabetic heart using preclinical animal AMI models
Several treatments for targeting inflammation to protect the diabetic heart have been tested in preclinical animal AMI models (see Table 1 for the summary).
Treatments with known anti-inflammatory targets
IL-10 is a potent anti-inflammatory cytokine broadly expressed by both adaptive and innate immune cells, which represses excessive inflammatory responses and maintains tissue homeostasis (Saraiva and O’Garra, 2010; Ouyang et al., 2011). Systematic treatment of IL-10 was shown to provide cardioprotection in diabetic mice, resulting in improved cardiac function and reduced infarct size by reducing cardiomyocyte apoptosis, reducing CD68+ cells infiltration in the border zone of the infarcted heart, and decreasing cardiac inflammatory gene expression (Gupta et al., 2020). The proposed mechanism involves IL-10 upregulation of the heme clearance pathways.
BAY11-7082 is a small molecule shown to directly suppress NLRP3 ATPase activity (Pagliaro and Penna, 2023). BAY11-7082 treatment 10 minutes before reperfusion reduced the infarct size in diabetic rats subjected to acute myocardial IRI (Qiu et al., 2017). BAY11-7082 decreased inflammasome-mediated pyroptotic cell death, reduced NLRP3, ASC, and caspase-1 protein levels, and reduced IL-1β production in diabetic rat hearts following IRI. In diabetic Goto-Kakizaki rats with AMI, the neutralizing antibody gevokizumab targeting IL-1β given one hour following reperfusion and continued for 90 days, reduced infarct size, limited the impairment in LV fractional shortening, decreased LV weight, collagen density, and delayed the progression to heart failure (Harouki et al., 2017).
The P2X7 receptor belongs to the ligand-gated ion channel P2X subfamily of purine-based P2 receptors, which is activated by extracellular ATP and activates NLRP3 inflammasome (Virgilio et al., 2017; Savio et al., 2018). The P2X7 receptor has a firmly established role in multiple inflammatory and immune responses. P2X7 was increased in expression after induction of DM in mouse models and further increased in DM mice after AMI (Wu et al., 2023). P2X7 receptor antagonists were then shown to alleviate myocardial IRI in diabetic mice. Pre-treatment with brilliant blue G (BBG) before AMI or acute treatment with A438079 30 minutes after reperfusion reduced the infarct size and improved ventricular systolic function in diabetic mice 24 hours after AMI. BBG and A438079 reduced NLRP3, caspase-1, and IL-1β protein production levels, decreased myocardial apoptosis, and increased the expression of cleaved caspase-3.
Treatments with broad-spectrum anti-inflammatory
Many natural agents and flavonoids have been reported to provide cardioprotection. Luteolin is a polyphenolic flavonoid widely present in many fruits and vegetables and has been suggested to have anti-inflammatory and antioxidant properties (López-Lázaro, 2009). Pre-treatment of luteolin for three days before acute myocardial ischemia/reperfusion (MI/R) reduced the infarct size of the diabetic rat hearts following MI/R (Sun et al., 2012). Luteolin reduced inflammatory responses, including reducing lactate dehydrogenase (LDH) release, leukocyte infiltration, and cytokine levels (myeloperoxidase, IL-6, IL-1a, TNF-α); enhanced LV ejection fraction and decreased myocardial apoptosis by decreasing caspase-3 and B-cell lymphoma 2 (Bcl-2)-associated agonist of cell death (BAD) protein, while increasing Bcl-2 and phosphorylated BAD. It was suggested that luteolin acts by activating the phosphoinositide 3-kinase/Akt pathway. Subsequent studies found pretreatment with luteolin protects diabetic rat hearts against IRI by enhancing endothelial nitric oxide synthase-mediated S-nitrosylation of Kelch-like ECH-associated protein 1 (Keap1), with upregulation of nuclear factor erythroid 2-related factor 2 (Nrf2) and the Nrf2-related antioxidative signaling pathway (Xiao et al., 2019).
Intermedin (IMD) belongs to the calcitonin gene-related peptide (CGRP) superfamily, with peptide fragment IMD1-53 generated by proteolytic cleavage (Roh et al., 2004). IMD has been suggested to have protective effects in isolated rat hearts (Yang et al., 2005). IMD levels were increased during IRI in diabetic rat hearts compared to sham-operated diabetic hearts (Li et al., 2013). Augmentation of IMD with IMD1-53 administration surprisingly had a cardioprotective response. IMD1-53 treatment significantly reduced infarct size in diabetic rats and showed anti-inflammatory effects by reducing TNF-α, IL-6, IL-1β, serum creatine kinase-MB (CK-MB), and LDH levels. IMD1-53 also attenuated malondialdehyde levels and decreased caspase-3 and apoptosis regulator BAX expression while increasing the anti-apoptotic Bcl-2 expression level.
Pistachios have been demonstrated to reduce insulin and glucose levels in patients. It was reported to reduce IRI in a streptozotocin (STZ)-induced diabetic rat of acute MI/R model by modulating inflammation (Di Paola et al., 2018). Pre-treatment of raw pistachios 18 hours before acute MI/R reduced infarct size, neutrophil infiltration, cytokines productions (e.g., TNF-α and IL-1β), adhesion molecules (e.g., intercellular cell adhesion molecule 1 and P-selectin), and expression of NF-κB and BAX. However, these effects were only investigated in an acute MI/R diabetic rat model after 30 minutes of ischemia and two hours of reperfusion. Thus, further studies of longer reperfusion duration are required.
Puerarin, an active ingredient of puerarin, was shown to improve cardiac function in rat models of MI and demonstrated protective effects in diabetic rat aorta (Ai et al., 2015; Li et al., 2016). Treatment of puerarin in diabetic IRI rats for four weeks markedly reduced the infarct size, increased LV developed pressure, LV end-systolic interior dimension, and the LV end-diastolic interior dimension (Guo et al., 2018). Serum cytokine TNF-α and IL-6 levels and NF-kB protein levels were significantly reduced by puerarin. Puerarin also activated the expression of vascular endothelial growth factor A (VEGFA) and angiotensin (Ang)-I, increased nitric oxide production, and reduced caspase-3 activity. This suggests that puerarin may reduce inflammation and oxidative stress by inhibiting NF-kB, upregulating the VEGFA/Ang-1 pathway, and suppressing apoptosis in diabetic rat hearts.
Pterostilbene (PT) is a naturally occurring demethylated analog of resveratrol and is most abundant in blueberries and known to have anti-inflammatory and anti-oxidative effects (Remsberg et al., 2008). Pre-treatment with PT significantly reduced infarct size in diabetic rats subjected to acute MI/R. PT also reduced myocardial apoptosis, plasma CK-MB level, and LDH release while increasing the Bcl-2/BAX ratio and reducing the cleaved caspase-3/caspase-3 ratio. PT pretreatment significantly increased the phosphorylation level of 5’ AMP-activated protein kinase (AMPK), suggesting AMPK activation plays a role in the activities of PT (Kosuru et al., 2018).
Troxerutin (TXR), or vitamin P4, is a derivative of the glucosidal natural bioflavonoid. It has broad-spectrum anti-inflammatory and anti-oxidative effects in rat models of kidney injury and anti-apoptotic potential against myocardial IRI in diabetic rats (Fan et al., 2009; Mokhtari et al., 2015). Pretreatment of diabetic rats with TXR reduced LDH release and inflammatory cytokine levels, including TNF-α and IL-1β following IRI (Najafi et al., 2018).
Polydatin (PD) is a natural hydroxyl-diphenyl ethylene compound isolated from the perennial herbage Polygonum cuspidatum Sieb. et Zucc. (Yu et al., 2018). It has been shown to have broad-spectrum anti-inflammatory activities and to promote autophagic flux and myocardial Ca2+ handling during IRI. Pre-treatment with polydatin ameliorated IRI, demonstrated by the reduced infarct size of diabetic rats after 30 minutes of ischemia and six hours of reperfusion. Polydatin also preserved cardiac function by increasing LV systolic pressure and reducing myocardial apoptosis and cleaved caspase-3 expression. The authors noted that polydatin administration restored the myocardial Notch1/Hes1 signaling pathway and enhanced Akt signaling by increasing phosphorylation.
Resveratrol (RVS) is a polyphenolic compound found in grapes, wines, peanuts, and many fruits and vegetables. RVS was shown to reduce NLRP3 inflammasome activation and caspase-1, as well as cytokine levels, including IL-1β and IL-18, in rat models of cerebral IRI (He et al., 2017). Pre-treatment with RVS limited the infarct size in diabetic rat hearts, reduced TNF-α and IL-6 serum levels, and upregulated the autophagy markers Beclin1 and light chain 3-II (LC3-II) (Qu et al., 2019).
Azathioprine (AZA), a widely used immunomodulator and immunosuppressant after transplantation, has been tested under many inflammatory conditions, such as multiple sclerosis and inflammatory bowel disease (Fraser et al., 2002; Casetta et al., 2007). It is a purine analog and inhibits DNA production, thus exerting inhibitory effects on leukocyte proliferation (Chavez-Alvarez et al., 2020). Pretreatment with AZA five days before acute MI/R reduced the infarct size of diabetic rat hearts (Lu et al., 2021). AZA also reduced levels of acute myocardial injury markers, such as CK-MB and myeloperoxidase, and oxidative stress markers, such as malondialdehyde and superoxide dismutase (SOD), in diabetic rat hearts following MI/R. AZA pretreatment decreased apoptosis by reducing the expression levels of caspase-8, caspase-3, and BAX while increasing Bcl-2 levels. Moreover, inflammatory Toll-like receptor 4 (TLR4) and TNF-α expression were also lower in the AZA pretreatment group.
Besides these pharmacological treatments, anti-diabetic drugs such as the sodium-glucose linked transporter 2 inhibitors (SGLT2-I) have also been investigated for their anti-inflammatory effects in diabetic rodent models of AMI. Canagliflozin, dapagliflozin, and empagliflozin have been reported to exert cardioprotection in animal models of AMI through improved cardiac function, reduced infarct size, and attenuation of heart failure development post-MI (Andreadou et al., 2020). The anti-inflammatory mechanisms suggested are diverse and may act by inhibiting the Na+/H+ exchanger, reducing intracellular Na+ and Ca2+, activating signal transducer and activator of transcription 3 and AMPK, inhibiting CamKII, and reducing inflammation and oxidative stress.
However, in many of the above diabetic murine models of acute MI/R, mice received short durations of reperfusion (less than 24 hours). This duration might be too short to determine the cardioprotective efficacy of these therapies, and longer durations of reperfusion are suggested to further investigate the benefits of these inflammatory therapies.
Clinical trials targeting inflammation in the diabetic heart against acute myocardial IRI
In the clinical setting of AMI patients undergoing reperfusion by percutaneous coronary intervention (PCI), the window of cardioprotection remains limited to the acute reperfusion period, which is less accessible clinically given the time pressure to reperfuse as soon as possible. The finding that administering anti-inflammatory agents, such as phosphoinositide 3-kinase -γ/δ inhibitors, up to three hours after myocardial reperfusion may make the translation of cardioprotective therapies more feasible (Doukas et al., 2006).
In patients with ST-segment elevation myocardial infarction (STEMI), the degree of the inflammatory response following AMI is a strong predictor of adverse LV remodeling (Seropian et al., 2014). Modulation of the inflammatory response represents a promising target for intervention. Despite encouraging results of anti-inflammatory treatments in pre-clinical models, only limited beneficial effects have been established in clinical studies. Table 2 highlights recent clinical trials employing anti-inflammatory therapies in STEMI patients, which include patients with DM.
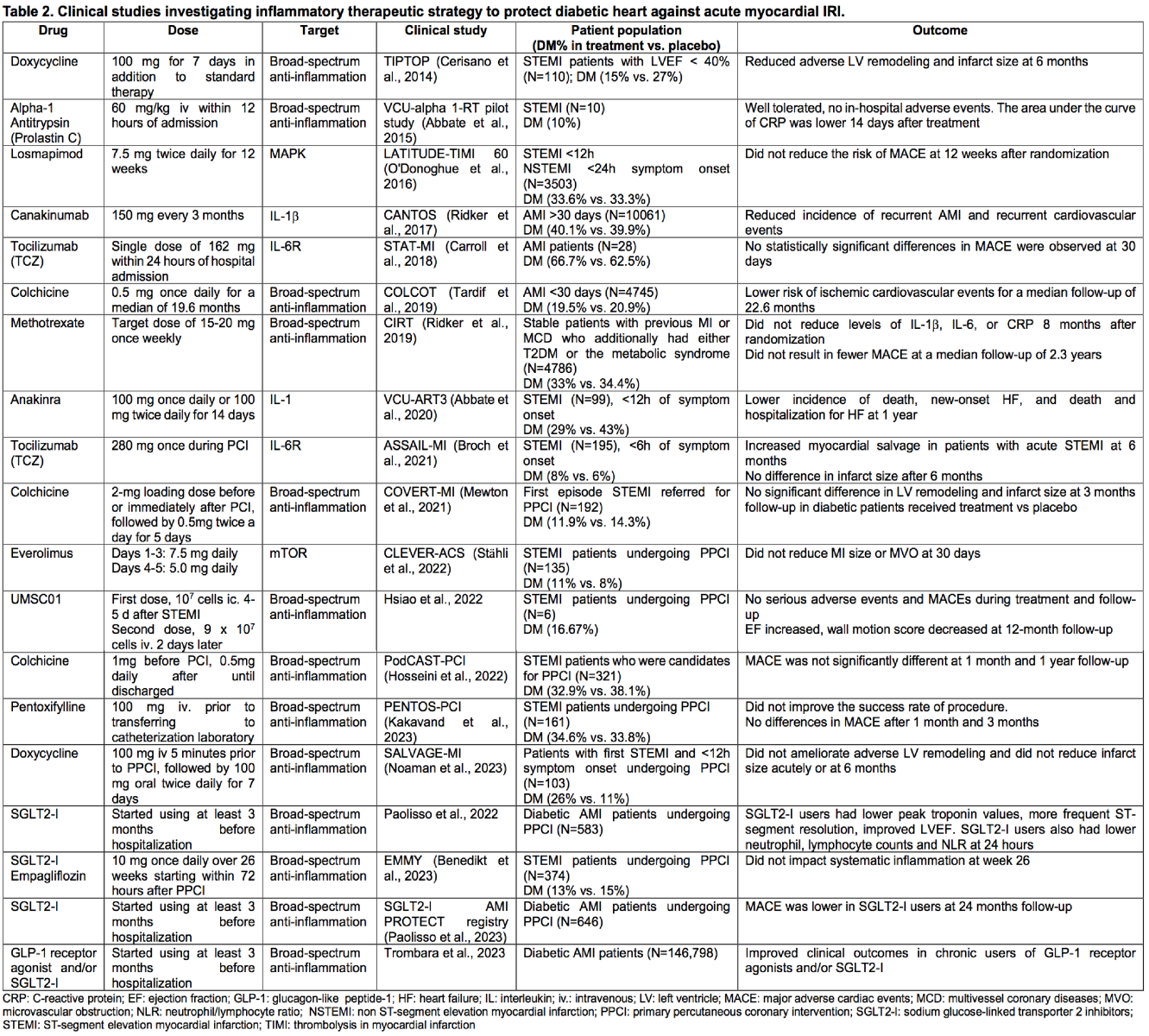
Treatments with known anti-inflammatory targets
The CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) trial with 10,061 patients with myocardial infarction was the largest phase 3 clinical trial. The CANTOS trial showed that canakinumab, a neutralizing monoclonal antibody, directly targeted inflammatory IL-1β and reduced cardiovascular event rates (Ridker et al., 2017). Study participants were followed for a median of 3.7 years, and the treatment group experienced a 15% reduction in major adverse cardiovascular events (MACE). This marked the first phase 3 clinical trial results that validated inflammation as a viable target for reducing MACE in AMI patients. However, in this study, the anti-inflammatory agents were given several days after AMI, so they did not target acute myocardial IRI.
Tocilizumab (TCZ) is a humanized monoclonal antibody that binds to the IL-6 receptor to block its signal transmission. TCZ has been shown to reduce both local and systemic IL-6 levels and is approved in various countries to treat rheumatoid arthritis (Carroll et al., 2018). TCZ was investigated in the STAT-MI (Short-term Application of Tocilizumab during Myocardial Infarction) trial as the first study to examine its benefits in AMI patients. Twenty-eight AMI patients were administered a single dose of TCZ as adjunctive AMI therapy within 24 hours of admission. While no statistically significant differences in MACE were noted 30 days after randomization, the authors noted no adverse events or safety signals. MACE was defined as recurrent MI, development of a new arrhythmia, new septal/valve rupture, evidence of dissection, pericarditis, or tamponade. However, the study had a small sample size, and the placebo was not matched. In the ASSAIL-MI (Assessing the effect of Anti-IL-6 treatment in MI) trial of 195 acute STEMI patients within six hours of symptom onset, a single dose of TCZ during PCI improved myocardial salvage and reduced the extent of microvascular obstruction, but there was no significant difference in the final infarct size at six months (Broch et al., 2021). However, the effects seemed limited to patients with symptom onset more than three hours before PCI, which would be expected given that the severity of acute myocardial IRI may be limited in patients presenting within three hours of symptom onset.
In the VCUART3 (The Virginia Commonwealth University-Anakinra Remodeling Trial-3) trial, IL-1 blockade with anakinra administered for 14 days in STEMI patients significantly reduced the systemic inflammatory response, incidence of death, new-onset HF or death, and HF hospitalizations compared to placebo at one-year follow-up (Abbate et al., 2020). Though there were no differences in adverse LV remodeling and systolic function, including LV end-systolic volume and LV ejection fraction (LVEF), this supports the potential clinical benefit of IL-1 blockade in patients with AMI.
The p38 MAPK is an intracellular kinase expressed in multiple cells, involved in several biological processes, including inflammation (Martin et al., 2012). MAPK activation amplifies inflammatory responses through enhanced release of TNF-α, IL-1β, IL-6, and metalloproteinases. Losmapimod, a selective, reversible, and competitive inhibitor of p38 MAPK (O’Donoghue et al., 2016), did not, however, reduce the risk of cardiovascular death or MI after 12 weeks of treatment in patients hospitalized with AMI in the LATITUDE-TIMI (The Losmapimod to Inhibit p38 MAP Kinase as a Therapeutic Target and Modify Outcomes After an Acute Coronary Syndrome–Thrombolysis in Myocaridal Infarction) 60 phase 3 clinical trial.
The CLEVER-ACS (Controlled-Level EVERolimus in Acute Coronary Syndrome) trial was a proof-of-concept trial to test whether inhibition of the mammalian target of rapamycin (mTOR) could attenuate the inflammatory responses in STEMI patients undergoing PCI (Stähli et al., 2022). The trial employed early inhibition of mTOR with everolimus, tailored to target the early pro-inflammatory phase post-AMI and to avoid interference with the healing phase. However, treatment with everolimus for five days post-AMI did not reduce the MI size, adverse LV remodeling, or microvascular obstruction (MVO) assessed by cardiac magnetic resonance imaging (CMR) at 30 days compared to the placebo group. It is suggested that the mTOR pathway may play a less important role in the pathogenesis of LV remodeling.
Treatments with broad-spectrum anti-inflammatory agents
Colchicine is an inexpensive, orally administered medication with broad-spectrum anti-inflammatory activity, often used to treat gout (Tardif et al., 2019; Opstal et al., 2020). In the COLCOT (Colchicine Cardiovascular Outcomes) trial, among 4,745 patients with AMI of less than 30 days, low-dose colchicine treatment led to a significantly lower risk of ischemic cardiovascular events than placebo, including death from cardiovascular causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalization for angina leading to coronary revascularization (Tardif et al., 2019). Contrary to the COLCOT trial, in the COVERT-MI (Colchicine for Left Ventricular Infarct Size Treatment in Acute Myocardial Infarction) trial of 192 STEMI patients, high-dose colchicine given at the time of reperfusion did not reduce infarct size compared to placebo at three months (Mewton et al., 2021). The diabetic STEMI patients showed no significant differences in LV remodeling compared to placebo. In addition, pretreatment with colchicine failed to improve patient outcomes in the PodCAST-PCI (The Preprocedural Colchicine in Patients with Acute ST-elevation Myocardial Infarction Undergoing Percutaneous Coronary Intervention) trial (Hosseini et al., 2022). The trial included 321 STEMI patients undergoing primary percutaneous coronary intervention (PPCI), in which low-dose colchicine was administered immediately before or immediately after PPCI and once daily after the procedure until discharge. Colchicine pretreatment did not reduce MACE events at one month or one year, including cardiac death, nonfatal MI, target lesion revascularization, target vessel revascularization, HF-related hospitalization, and stroke post-procedurally.
The CIRT trial (Cardiovascular Inflammation Reduction Trial), which was planned in parallel with the CANTOS trial, used an alternative approach to inhibiting inflammation using low-dose methotrexate (Ridker et al., 2019). Low-dose methotrexate is inexpensive, effective, and widely used to treat inflammatory conditions, including rheumatoid arthritis. However, among 4786 patients with previous MI or multivessel coronary disease who additionally had either T2DM or the metabolic syndrome, low-dose methotrexate treatment once weekly for eight months did not reduce levels of IL-1β, IL-6, or C-reactive protein (CRP). It was not associated with fewer cardiovascular events than placebo, including nonfatal MI, nonfatal stroke, or cardiovascular death, in the median follow-up of 2.3 years.
Pentoxifylline is a methylxanthine derivative with known broad-spectrum anti-inflammatory, antioxidant, and vasodilator properties, which might be a promising agent to reduce IRI (Kakavand et al., 2023). However, in the PENTOS-PCI (Evaluating the Role of Intravenous Pentoxifylline Administration on Primary Percutaneous Coronary Intervention Success Rate in Patients with ST-elevation Myocardial Infarction) trial, where a single dose of intravenous pentoxifylline was evaluated for efficacy and safety in acute STEMI patients undergoing PPCI, pentoxifylline did not improve the primary endpoint indexing PCI’s success rate, as measured by thrombolysis in myocardial infarction flow grade 3. Pentoxifylline also did not improve MACE 30 days and three months after randomization compared to placebo.
Another broad-spectrum anti-inflammatory therapy is doxycycline, which has been shown to inhibit metalloproteinases (MMPs). MMP activation contributes to LV remodeling, and pharmacological MMP inhibition has shown a potential cardioprotective effect in isolated animal hearts subjected to IRI (Cheung et al., 2000). In the TIPTOP (Early Short-term Doxycycline Therapy in Patients with Acute Myocardial Infarction and Left Ventricular Dysfunction to Prevent the Ominous Progression to Adverse Remodeling) trial, short-term doxycycline treatment was given immediately after PPCI and every 12 hours for seven days to 110 STEMI patients with LV dysfunctions (Cerisano et al., 2014). The percentage change from baseline to six-month follow-up, LV end-diastolic function index (%Δ LVEDVi) was significantly lower in the doxycycline group than in the placebo. Doxycycline also increased the ΔLVEF, reduced the infarct size measured by single photon emission computed tomography scan, and reduced MACE events (death, MI, congestive HF, and stroke) at six months compared to placebo. Later, in the SALVAGE-MI (The Upstram Doxycycline in ST-elevation Myocardial Infarction: Targeting Infarct Healing and Modulation trial) trial in 103 STEMI patients, doxycycline was given before PPCI, followed by seven days. Contrary to the TIPTOP trial, the treatment did not reduce adverse LV remodeling (%Δ LVEDVi) and even worsened the final infarct size measured by CMR compared to the placebo at six months (Noaman et al., 2023). Clinical outcomes (mortality, HF re-admissions) at six months were comparable between the two groups. These results cast doubts on the actual benefits of doxycycline and, more broadly, MMP inhibition in patients with AMI.
Alpha-1 antitrypsin is a serine protease inhibitor found abundant in plasma with broad anti-inflammatory effects on caspase-1 and inflammatory cytokine IL-1β, which has demonstrated cardioprotection in mouse models of IRI (Toldo et al., 2011; Mauro et al., 2017). Human plasma-derived alpha-1 antitrypsin (Prolastin C) was then demonstrated in the VCU-Apha 1-RT pilot study for safety and tolerance in 10 clinically stable STEMI patients (Abbate et al., 2015). Prolastin C given as a single infusion within 12 hours of revascularization was safe and well-tolerated. The prolastin C-treated group had a smaller area under the curve for CRP levels at follow-up on day 14 compared to historical placebo controls, demonstrating potential anti-inflammatory effects.
Besides small molecules and antibody therapies, cell-based therapies have also been intensively studied recently, including mesenchymal stem cells (MSCs), which are considered a promising therapy for AMI (Shafei et al., 2017; Wagner et al., 2020). The pilot trial with umbilical cord-derived mesenchymal stem cells (UMSC01) is the first human study to assess the efficacy of cell-based therapy in post-AMI cardiac repair (Hsiao et al., 2022). Six patients with their first STEMI episode undergoing PPCI were recruited and were followed up for 12 months. Intracoronary infusion of the first dose of UMSC01 was performed four to five days via the index culprit artery after successful revascularization, and intravenous infusion of the second dose was carried out two days later. No serious adverse events or MACEs were observed during the study period. At 12-month follow-up, the stroke volume and LVEF increased, while the wall motion score decreased as measured by CMR. This is the first phase 1 pilot study to demonstrate the safety and proof-of-concept for the feasibility of cell-based therapies targeting IRI and inflammation following AMI. Larger randomized and placebo-controlled studies are thus encouraged to demonstrate the efficacy of MSCs and cell-based therapies.
Besides the abovementioned therapies, many anti-diabetic medications have also been reported to influence inflammation in DM patients with MI. In a score-matched analysis of DM patients (defined by fasting blood glucose >7 mmol/L or 126 mg/dL, regardless of the duration of disease or need for glucose-lowering medications) presented with STEMI, ongoing use of metformin before the event did not result in significant differences in CK-MB, troponin T, or LVEF (Basnet et al., 2015). Investigation of metformin on IRI in pre-clinical and clinical patients has further been carefully detailed in the following review paper (Higgins et al., 2019). It is suggested that beneficial effects may depend on dosage, timing, and the condition of patients receiving metformin treatment.
Sodium glucose-linked transporter 2 inhibitors (SGLT2-I) are also thought to have beneficial effects on inflammation and oxidative stress in addition to their anti-hyperglycemic properties and have been investigated for cardioprotective effects in multiple studies (Chen et al., 2022). In a multicenter international registry involving 583 diabetic AMI patients undergoing PPCI, SGLT2-I users of at least three months before hospitalization were reported to have improved LVEF, ST resolution, and inflammatory indices, including lower neutrophil and lymphocyte levels at 24 hours (Paolisso et al., 2022). This was the first study to report the cardioprotective role of SGLT2-I therapy in diabetic patients with AMI, thus suggesting potential beneficial effects of SGLT2-I therapy and supporting further investigation in larger cohosts. The first report investigating long-term outcomes in T2DM patients admitted with AMI showed that SGLT2-I users had lower in-hospital mortality, MACE events including cardiovascular mortality, and HF hospitalization at 24 months follow-up (Paolisso et al., 2023). However, the results have still been controversial. In another randomized, multicentric, double-blind, and placebo-controlled trial, the EMMY (Impact of Empagliflozin on Cardiac Function and Biomarkers of Heart Failure in Patients with Acute Myocardial Infarction) trial, of 476 STEMI patients undergoing PPCI, Empagliflozin treatment at week 26 did not impact systemic inflammation, including IL-6, high sensitive CRP, neutrophil count, leukocyte count, and neutrophil/lymphocyte ratio (Benedikt et al., 2023).
Other anti-diabetic drugs, such as glucagon-like peptide-1 receptor agonists (GLP-1RA), may also have cardioprotective effects. Among 146,798 diabetic AMI patients, chronic therapy with GLP-1 receptor agonists and/or SGLT2-I had beneficial effects on clinical outcomes, including in-hospital mortality, acute HF, and acute kidney injury (Trombara et al., 2023). DM patients showed an intermediate risk between that of non-DM patients and DM patients who did not take these drugs. Other trials investigating SGLT2-I and GLP-1RA effects on cardiovascular events have been extensively studied in the following systematic review (Lee et al., 2020). The two classes of drugs may improve blood glucose and reduce clinical adverse events, and thus, further investigations on the mechanisms are encouraged.
The conflicting results of different clinical trials thus highlight the complex nature of targeting inflammation post-AMI, and the timing of interventions and the pharmacokinetics are also crucial. These challenges need to be tackled to identify effective therapies to reduce infarct size and limit adverse LV remodeling post-AMI. Since inflammation acts in multiple redundant and interconnected pathways, identifying the appropriate target is challenging, and it may be difficult to predict clinical efficacy before phase 3 testing. Moreover, these trials also highlight the importance of understanding the diversity of inflammatory pathways and exploring approaches to their inhibition. Until now, most of the treatments remain expensive, including canakinumab and TCZ (Ma and Chen, 2021). Canakinumab may also have serious adverse effects, and TCZ affects lipid levels. Its long-term effects and safety need further evaluation.
Conclusion
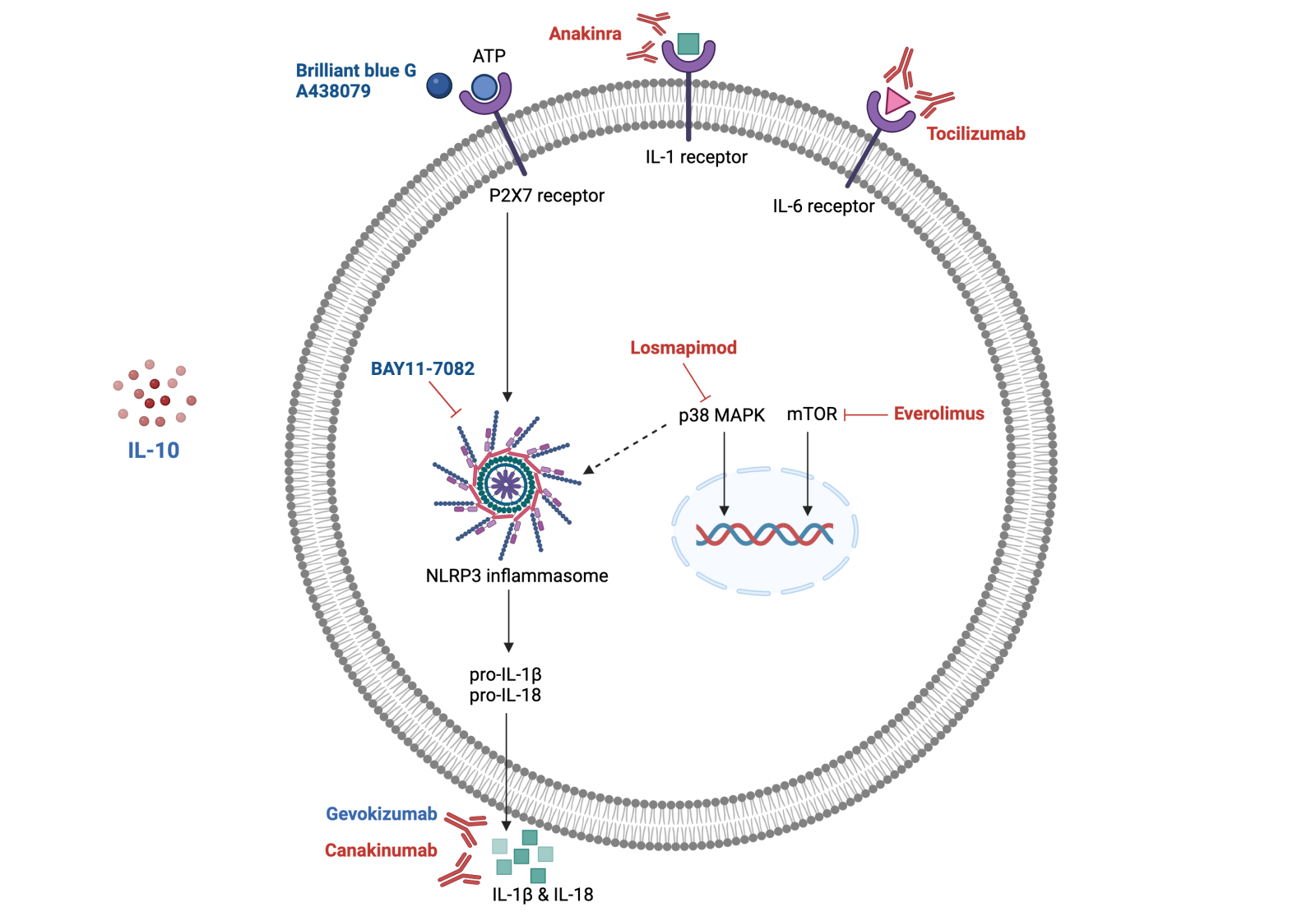
Currently, the pharmacological treatment and interventions for diabetic AMI patients remain limited and ineffective. The presence of diabetes aggravates inflammation in the AMI myocardium, leading to increased infarct size and worse outcomes following AMI. Due to the complexity of the inflammation and immune systems following AMI, targeted immunotherapeutic strategies are slow to emerge. However, the success of the CANTOS trial has provided proof-of-concept for the feasibility of immunotherapy. The emergence of unbiased, high-throughput single-omics, spatial, and temporal technologies will facilitate our understanding of the immune cell populations following AMI and novel signaling pathways for therapeutic applications. Pre-clinical and clinical therapies targeting inflammation against MI/R in diabetic hearts with known targets are summarized in Figure 1.
In a new window | Download PPT
Figure 1. Therapies targeting inflammation following AMI in diabetic heart in preclinical studies (highlighted in blue) and clinical studies (highlighted in red). Inflammation-targeting therapies following myocardial IRI often target cytokine receptors (IL-1, IL-6 receptors), reducing pro-inflammatory cytokine production post-AMI. Other therapies target the P2X7 receptor or p38 MAPK, which converge on the NLRP3 inflammasome that triggers the inflammation response. Immunotherapies often target the pro-inflammatory cytokines directly, including IL-1β and IL-18. IL-1, 1β, 6, 10, 18: interleukin 1, 1β, 6, 10, 18; p38 MAPK: p38 mitogen-activated protein kinases; mTOR: mammalian target of rapamycin; P2X7 receptor: P2X purinoceptor 7; NLRP3 inflammasome: NLR family pyrin domain containing 3 inflammasome.
Acknowledgments
Conflicts of interest
All authors have no relevant conflicts or disclosures.
References
Linh Chi Dam1,2
1Cardiovascular and Metabolic Disorder Program, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
William Boisvert3
3Center for Cardiovascular Research, John A. Burns School of Medicine, University of Hawaii, USA.
Sauri Hernandez-Resendiz1,2
1Cardiovascular and Metabolic Disorder Program, Duke-NUS Medical School, Singapore. 2National Heart Research Institute Singapore, National Heart Centre, Singapore.
Corresponding author:
Sauri Hernandez-Resendiz
Email: sauri.hdz@duke-nus.edu.sg

In a new window | Download PPT
Figure 1. Therapies targeting inflammation following AMI in diabetic heart in preclinical studies (highlighted in blue) and clinical studies (highlighted in red). Inflammation-targeting therapies following myocardial IRI often target cytokine receptors (IL-1, IL-6 receptors), reducing pro-inflammatory cytokine production post-AMI. Other therapies target the P2X7 receptor or p38 MAPK, which converge on the NLRP3 inflammasome that triggers the inflammation response. Immunotherapies often target the pro-inflammatory cytokines directly, including IL-1β and IL-18. IL-1, 1β, 6, 10, 18: interleukin 1, 1β, 6, 10, 18; p38 MAPK: p38 mitogen-activated protein kinases; mTOR: mammalian target of rapamycin; P2X7 receptor: P2X purinoceptor 7; NLRP3 inflammasome: NLR family pyrin domain containing 3 inflammasome.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4327 | 6 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA