Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Stroke gets a cold treatment: Hypothermic recombinant tissue plasminogen activator improves neuroprotection
Time:2024-05-07
Number:4784
Maximillian C. Borlongan1, Mia C. Borlongan2, Yumin Luo3,4,5
Author Affiliations


- 1Vanderbilt University, Nashville, TN.
- 2California Northstate University, Rancho Cordova, CA.
- 3Institute of Cerebrovascular Diseases Research, Department of Radiology and Nuclear Medicine, Xuanwu Hospital of Capital Medical University, Beijing, China.
- 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Diseases, Beijing, China. 5Beijing Institute for Brain Disorders, Beijing, China.
- 5Beijing Institute for Brain Disorders, Beijing, China.
Conditioning Medicine 2023. 6(5): 160-164.
Abstract
Intra-arterial injection of recombinant tissue plasminogen activator (rtPA) within the 4.5-hour window of ischemic stroke onset improves the prognosis in patients. However, recanalization-induced negative consequences, such as vascular edema and hemorrhagic transformation, accompany rtPA treatment. Finding an adjunctive therapy with tPA may reduce such adverse events. Preclinical and clinical evidence demonstrates that selective brain cooling or hypothermia promotes neuroprotection in stroke. Building upon this concept of combined thrombolysis and hypothermia, we recently explored intra-arterial hypothermic rtPA in adult rats exposed to experimental stroke. Our findings showed that hypothermic rtPA reduced neurological deficits, cerebral infarction, and hemorrhagic transformation in stroke rats. By dampening blood-brain barrier leakage, hypothermic rtPA improved energy metabolism and reduced inflammation. Here, we highlight the robust therapeutic mechanisms induced by hypothermic rtPA to optimize its eventual application as a treatment for ischemic stroke.
Keywords: Cerebral ischemia, thrombolysis, hypothermia, blood-brain barrier, inflammation
Abstract
Intra-arterial injection of recombinant tissue plasminogen activator (rtPA) within the 4.5-hour window of ischemic stroke onset improves the prognosis in patients. However, recanalization-induced negative consequences, such as vascular edema and hemorrhagic transformation, accompany rtPA treatment. Finding an adjunctive therapy with tPA may reduce such adverse events. Preclinical and clinical evidence demonstrates that selective brain cooling or hypothermia promotes neuroprotection in stroke. Building upon this concept of combined thrombolysis and hypothermia, we recently explored intra-arterial hypothermic rtPA in adult rats exposed to experimental stroke. Our findings showed that hypothermic rtPA reduced neurological deficits, cerebral infarction, and hemorrhagic transformation in stroke rats. By dampening blood-brain barrier leakage, hypothermic rtPA improved energy metabolism and reduced inflammation. Here, we highlight the robust therapeutic mechanisms induced by hypothermic rtPA to optimize its eventual application as a treatment for ischemic stroke.
Keywords: Cerebral ischemia, thrombolysis, hypothermia, blood-brain barrier, inflammation
Highlights
Neuroprotection is almost synonymous with conditioning medicine. The concept entails conditioning the brain from impending injury. While stroke has been traditionally considered an acute injury, subsequent secondary cell death ensues after the primary ischemic injury. Accordingly, preparing the stroke brain for this impending cascade of cell death events is paramount for improved functional outcome. Here, we review relevant studies, highlighting our recent report on combined hypothermia and recombinant tissue plasminogen activator (rtPA) via intra-arterial injection of hypothermic rTPA as a novel approach in conditioning the brain from the consequential recanalization-induced adverse effects of vascular edema and hemorrhagic transformation.
Current status of stroke neuroprotection
The failure of several neuroprotective agents against stroke has produced a dismal record for finding new effective treatments for stroke; thereby, finding effective stroke treatments remain a significant unmet clinical need. Indeed, only one neuroprotective strategy has been successful, namely intra-aterial delivery of recombinant tissue plasminogen activator (rtPA) (Neuhaus et al., 2017; Renu et al., 2022). Unfortunately, rtPA may be accompanied by adverse events, including vascular edema and hemorrhagic transformation, tapering rPA’s therapeutic functional outcomes in ischemic stroke (Sengeze et al., 2022; Li et al., 2020).
Several laboratory studies and clinical trials have explored the use of hypothermia in treating ischemic stroke, particularly in conjunction with other therapeutic interventions. As an ischemic stroke treatment, hypothermia has been utilized in neurological intensive care units (Wu et al., 2020). Clinical evidence indicates intra-arterial selective cooling is safe for stroke patients undergoing mechanical thrombectomy (Wu et al., 2020). In pre-clinical experiments in primates, the safety and efficacy of intra-arterial selective hypothermia have been demonstrated, particularly in protecting against brain damage induced by ischemia (Wu et al., 2018). In rat studies, intra-arterial hypothermic injection decreases cerebral infarction volume (Wang et al., 2022) and blood-brain barrier (BBB) damage (Liu et al., 2018) in animals with middle cerebral artery occlusion (MCAO). Recognizing that the major limitation of rtPA usage in ischemic stroke treatment is its consequential adverse events, specifically malignant cerebral edema and hemorrhagic transformation, finding an adjunctive therapy that reduces these side effects is likely to improve rtPA’s therapeutic functional outcomes.
To this end, we recently explored the potential benefits of hypothermic therapy with rtPA via intra-arterial injection of cold rtPA. The goal of this combined hypothermia and rtPA treatment was to mitigate these adverse events and improve neuroprotection against ischemic stroke. Our study assessed whether mild hypothermic rtPA can lessen brain injury arising from vascular recanalization treatment in ischemic stroke (Shi et al., 2021; Huang et al., 2023). This rationale for investigating the combined use of hypothermia and rtPA in ischemic stroke treatment is based on the bulk of evidence supporting the efficacy and safety of hypothermia and the need to address limitations associated with rtPA usage.
Combined hypothermia and rtPA
Our recent study (Huang et al., 2023) investigated the safety and feasibility of hypothermic rtPA, aiming to broaden the application scenarios of rtPA to benefit a broader range of patients. Specifically, the study sought to 1) evaluate the safety profile of selective hypothermic rtPA injection, which included assessing potential negative effects such as hemorrhagic transformation and malignant cerebral edema associated with rtPA application in combination with hypothermic therapy. Comprehensive monitoring and analysis were conducted to determine the occurrence and severity of adverse events; 2) assess the practicality and efficacy of selective hypothermic rtPA injection by examining the feasibility of administering hypothermic rtPA through intra-arterial infusion into the medial cerebral artery in focal cerebral ischemia. Efficacy endpoints focused on the extent of brain injury related to vascular recanalization treatment and the degree of neuroprotection against ischemic stroke; 3) investigate the mechanisms underlying the neuroprotective effect through preclinical stroke modeling and molecular analyses. By elucidating the mechanisms by which hypothermic rtPA injection exerts its neuroprotective effects, we began to appreciate its impact on key pathways involved in ischemic injury, such as inflammation, oxidative stress, and BBB integrity. Altogether, our impetus in addressing these objectives should provide evidence supporting the safety and efficacy of selective hypothermic rtPA injection as a therapeutic approach for ischemic stroke. Furthermore, insights gained into the underlying mechanisms may offer valuable information for optimizing treatment strategies and potentially enhancing outcomes for stroke patients.
In our study, adult Sprague-Dawley rats were subjected to the MCAO model (Huang et al., 2023). They received 2 mL of 4°C rtPA (0.07 mg/ml) via a modified PE-10 catheter (0.2-mm outer diameter and 0.1-mm inner diameter) inserted through the incision in the carotid artery to induce hypothermia as soon as reperfusion. Our results revealed that selective hypothermic rtPA reduced neurological deficits, cerebral infarction, and hemorrhagic transformation in stroke rats. Hypothermic rtPA improved energy metabolism and reduced inflammation by dampening blood-brain barrier leakage. First, we found that hypothermic rtPA decreased brain temperature, glucose metabolism, and cerebral infarct volume in MCAO rats. We detected that the cortex and basal ganglia temperature declined more significantly after hypothermic rtPA injection than the normothermic rtPA injection, with the hypothermic effect lasting for 20 minutes. Additionally, the hypothermic treatment slightly decreased rectal temperature. The 18F-fluorodeoxyglucose positron emission tomography-magnetic resonance imaging scans at 24h and 72h after reperfusion revealed that the metabolic activity of 18F-fluorodeoxyglucose in the cerebrum decreased in the hypothermic rtPA-treated stroke rats but increased in the normothermic rtPA-injected stroke rats. Measurements of the infarct volume on T2-weighted images at 24h and 72h after reperfusion revealed that the infarct volume decreased in the hypothermic rtPA-treated stroke rats but again increased in the normothermic rtPA-injected stroke rats. Second, hypothermic rtPA reduced neurological deficits and limited hemorrhage transformation in MCAO rats. Less behavioral dysfunctions were noted in the Longa score, foot fault test, tape removal test, and modified neurological severity score in hypothermic rtPA-treated stroke rats compared to normothermic rtPA-injected stroke rats. Interestingly, Prussian blue+ cells were detected in the brains of the normothermic rtPA-injected stroke rats, indicating hemosiderin accumulation or hemorrhagic transformation in the stroke brain, which were rarely observed in the brain tissues of hypothermic rtPA-treated stroke rats. Third, hypothermic rtPA lessened BBB damage. Using Western blot after 72-h reperfusion, we detected increased levels of vasogenic edema proteins matrix metalloproteinase-9 and matrix metalloproteinase-2 in normothermic rtPA-injected stroke rats, which were suppressed in hypothermic rtPA-treated stroke rats. In further support of BBB integrity, the expression levels of tight junction proteins occludin and claudin5 increased in hypothermic rtPA-treated stroke rats compared to normothermic rtPA-injected stroke rats. Fourth, hypothermic rtPA dampened the expression of pro-inflammatory factors in the plasma of hypothermic rtPA-treated stroke rats. Although not statistically significant, the expression of chemokine (C-X-C motif) ligand 1, granulocyte-macrophage colony stimulating factor, and chemokine (C-C motif) ligand 2 trended toward near normal levels in hypothermic rtPA-treated stroke rats compared to normothermic rtPA-injected stroke rats. Moreover, the expression of fibrinolytic-related proteins urokinase-type plasminogen activator receptor (uPAR) and cellular fibronectin (cFn) in plasma and cerebrospinal fluid also trended toward near-normal levels in hypothermic rtPA-treated stroke rats compared to normothermic rtPA-injected stroke rats. Additionally, some evidence suggested hypothermic rtPA limited neutrophil infiltration in brain tissue 72 h after reperfusion. Fifth, hypothermic rtPA created a metabolomic signature that may serve as a biomarker of functional recovery of stroke rats in response to hypothermia and rtPA. In summary, the novelty of our study reveals that hypothermic rtPA reduced brain temperature, glucose metabolism, and cerebral infarction volume, ameliorated neurological deficits, limited hemorrhage transformation, diminished BBB damage, tapered the plasma level of pro-inflammatory factors, and produced a metabolomic biomarker for stroke recovery. The significance of this study is that the co-application of two stand-alone stroke treatments may promote improved therapeutic outcomes while circumventing adverse effects associated with each treatment.
Future directions and clinical implications: Contemplating hypothermic rtPA for clinical application
Our study (Huang et al., 2023) focused on rescuing the ischemic penumbra in cerebral ischemic injury, which we believe represents a crucial aspect of neuroprotection research. Despite scientific progress and clinical breakthroughs in vascular recanalization, many patients still face poor prognoses due to infarct core expansion, reduction of the ischemic penumbra, postoperative no-reflow, and reperfusion injury (Camara et al., 2021; Meinel et al., 2022; Richter et al., 2005; Li et al., 2021). Additionally, the risk of hemorrhagic transformation associated with delayed recanalization further complicates treatment outcomes (Bang et al., 2011). These clinical caveats solicit innovation and refinement of current stroke treatments.
Hypothermia has emerged as a promising therapeutic approach for protecting the ischemic penumbra from recanalization-associated adverse events (Choi, 2017). Clinical trials investigating hypothermia in patients undergoing recanalization have shown encouraging results (Chen et al., 2016). Intra-arterial mild hypothermia significantly reduces infarct volume without increasing adverse reactions (Piironen et al., 2014). Moreover, stroke patients treated with hypothermia exhibited improved functional outcomes, as indicated by positive trends in neurological scores at follow-up (Chen et al., 2016). Intravascular hypothermia also shows promise in improving neurological scores in stroke patients undergoing intravenous thrombolysis. Selective hypothermia therapy, particularly when initiated promptly to target the ischemic penumbra, appears effective when used as an adjunct to thrombolysis. Microcatheter infusion of cold saline prior to intravascular revascularization prompts immediate temperature reduction in localized brain areas, potentially enhancing the therapeutic benefits (Wu et al., 2020). This clinical evidence suggests the potential of hypothermia as an adjunctive therapy for stroke. Indeed, the integration of hypothermia therapy with recanalization procedures holds considerable promise for mitigating the adverse effects of ischemic stroke treatment and improving patient outcomes. Optimizing the clinical implementation of hypothermia as a neuroprotective strategy in ischemic stroke management warrants further research.
Our recent findings (Huang et al., 2023) provide the scientific basis for developing hypothermic rtPA as an innovative approach to enhance neuroprotection while reducing the thrombolytic adverse events of ischemic stroke treatment. By selectively delivering hypothermia concurrent with rtPA treatment into the medial cerebral artery territory, which is the ischemic penumbral region, we aimed to capitalize on the combined benefits of these therapies in arresting penumbral expansion. We demonstrated improved neuroprotection with the targeted injection of hypothermic rtPA into the medial cerebral artery region, suggesting enhanced neuroprotective effects on brain tissues compared to stand-alone therapies. We implicate the role of temperature and metabolic rate modulation by hypothermic rtPA, as evidenced by effectively reducing brain temperature and cerebral metabolism in stroke animals but circumventing any alterations in cerebral blood flow, altogether indicating precise modulation of physiological parameters. We also showed improved therapeutic functional outcomes in that hypothermic rtPA administration significantly improved neurological function in MCAO rats, suggesting potential benefits for functional recovery post-stroke. We also provided mechanistic evidence of preserving BBB integrity, which is critical for preventing further damage and maintaining cerebral homeostasis. With limited BBB damage, we achieved reduced adverse events, specifically lowering the incidence of hemorrhagic transformation, a serious complication of thrombolytic therapy. Accordingly, hypothermic rtPA treatment decreased recanalization-induced side effects, indicating an improved safety profile. Finally, we showed hypothermic rtPA suppressed deleterious inflammatory responses associated with cerebral ischemia, which could contribute to mitigating secondary injury cascades. Overall, our study provides compelling evidence for the efficacy and safety of hypothermic rtPA therapy in ischemic stroke management. These findings underscore the potential of combining hypothermia with thrombolytic treatment to optimize neuroprotection and improve outcomes for stroke patients. Additional lab-to-clinic research needs to optimize the dosing regimen of hypothermic rtPA therapy for its translation into clinical practice.
The concept of the ischemic penumbra, characterized by a "mismatch" between oxygen supply and demand, underscores the critical need for interventions to prevent its progression to irreversible damage (Leigh et al., 2018). Thrombolysis and recanalization therapies aim to preserve the penumbra, but their potential adverse events, such as hemorrhagic transformation, necessitate the exploration of adjunctive strategies. In ischemic brain injury, compromised cellular metabolism disrupts energy homeostasis, leading to cell dysfunction and, ultimately, apoptosis (Nakagawa et al., 2005; Capone et al., 2021). Hypothermia offers a promising approach by reducing the energy demands of cells under pathological conditions like ischemia. By maintaining an adequate energy supply for essential cellular processes, hypothermia can mitigate the initiation of cell death pathways. The use of PET-MR imaging in our study (Huang et al., 2023) to monitor glucose uptake and metabolism in rat brain tissue provided valuable insights into the effects of hypothermia on ischemic penumbra metabolism. The findings demonstrate that hypothermia effectively reduces metabolic activity in the stroke brain, decreasing neuronal activity and energy demand within the ischemic penumbra. This modulation of metabolic activity helps restore homeostasis by balancing oxygen exchanges in the penumbra, potentially preserving tissue viability and reducing the risk of progression to irreversible injury. Our observations highlight the therapeutic potential of hypothermia in mitigating metabolic dysfunction and preserving the ischemic penumbra (Huang et al., 2023). By targeting energy metabolism, hypothermia may offer a valuable adjunctive strategy to enhance the efficacy of thrombolysis and recanalization therapies while minimizing their adverse effects, ultimately improving outcomes for ischemic stroke patients.
The complex interplay between recanalization and reperfusion injury may benefit from combined hypothermia and rtPA treatment in ischemic stroke. A major challenge with recanalization and reperfusion is that even after successful recanalization, reperfusion injury may persist due to impaired neurovascular unit function and microthrombosis formation. This underscores the need for adjunctive therapies to mitigate these risks and optimize outcomes. In advancing hypothermic rtPA, the question arises whether the thrombolytic efficiency of rtPA remains unaltered under hypothermia. While there have been concerns about the temperature-dependent activity of rtPA, both in vitro studies and clinical trials suggest that the thrombolytic efficiency of rtPA is minimally affected by mild hypothermia (Rijken et al., 1990; Lees et al., 2011). Although there may be a slight decrease in catalytic activity with decreasing temperature, the overall effect on thrombolysis appears tolerable (Huang et al., 2023). In analyzing the neuroprotective effects of hypothermic rtPA, equally critical is monitoring its consequential actions on the adverse events associated with rtPA, particularly in reducing rtPA-associated side effects such as hemorrhagic transformation. By reducing the expression of inflammatory chemokines and up-regulating fibrinolysis-related proteins like uPAR and cFn, hypothermic rtPA treatment modulates the inflammatory response and fibrinolytic system without excessive activation, ultimately leading to reduced neutrophil infiltration into the brain tissue (Huang et al., 2023). Despite these positive outcomes, we must consider the limitations and future directions of hypothermic rtPA. We acknowledge the limitations of animal modeling in that, using the MCAO, we were unable to explore the dissolution effect of rtPA on microemboli (Huang et al., 2023). Future research could further investigate the mechanisms underlying the neuroprotective effects of hypothermic rtPA and its potential impact on microthrombosis resolution using the embolic clot stroke model. Nonetheless, our study contributes to our understanding of the therapeutic potential of hypothermic rtPA in ischemic stroke management, highlighting its ability to mitigate thrombolytic adverse events and modulate inflammatory responses while preserving fibrinolysis balance (Huang et al., 2023). Novel treatment strategies aimed at optimizing recanalization therapies, such as hypothermic rtPA, may improve outcomes for ischemic stroke patients. Nonetheless, the potential adverse effects and limitations of hypothermic rtPA need to be considered, which may include altered pharmacokinetics and pharmacodynamics of rtPA under reduced temperatures, the risk of systemic complications, or the challenges in clinical management of hypothermia. Accordingly, strategies designed to address these adverse effects and limitations should be explored to advance the safe and effective translation of hypothermic rtPA to the clinic. In particular, optimization of the timing, degree of cooling, duration of hypothermia, and how these factors interact with thrombolytic therapy will likely enhance patient outcomes post-stroke. By recognizing the laboratory methods associated with hypothermic rtPA, the development of clinical protocols for ischemic stroke treatment will benefit from the pharmacokinetics and pharmacodynamics of rtPA under reduced temperature; vice versa, understanding how hypothermia can be selectively targeted to specific regions of the brain as opposed to systemic cooling, will also need to be evaluated in both preclinical and clinical settings to better assess the safety and efficacy of hypothermic rtPA. Moving forward, contemplating how this combined treatment approach could be integrated into current stroke treatment algorithms and what further investigations are needed to validate and refine this strategy for broader clinical application should be pursued. Co-morbidity factors such as diabetes and hypertension should be assessed in tandem with hypothermic rtPA. Additionally, mechanical thrombectomy instead of rtPA should also be tested to fully identify the target stroke patient population.
Conclusions
In summary, our recent study represents a significant advancement in applying hypothermic rtPA therapy for ischemic stroke (Huang et al., 2023). The findings demonstrate the safety and efficacy of concurrent hypothermic treatment with rtPA, providing valuable insights into its potential clinical utility (Figure 1). Specifically, our data indicate that selective cooling combined with rtPA treatment is safe and effective in enhancing neuroprotection while mitigating the risk of hemorrhagic transformation in the MCAO rat model. This suggests that hypothermic rtPA therapy has the potential to improve outcomes for ischemic stroke patients by reducing adverse events while enhancing neuroprotective effects. In addition, our study introduces a novel approach to ischemic stroke treatment by combining hypothermia with rtPA therapy. This innovative strategy offers a promising alternative for improving the management of ischemic stroke and minimizing treatment-related complications. The findings provide preclinical validation of hypothermic rtPA therapy, laying the groundwork for further investigation and potential translation into clinical practice. By demonstrating safety and efficacy in animal models, the study sets the stage for future clinical trials to evaluate the feasibility and effectiveness of this treatment approach in human patients. The results of the study have important implications for clinical practice, suggesting that hypothermic rtPA therapy may represent a valuable therapeutic option for ischemic stroke patients. By enhancing neuroprotection and reducing adverse events, this approach has the potential to improve outcomes and enhance patient care in the management of ischemic stroke. That hypothermic rtPA therapy is a safe and effective ischemic stroke treatment warrants further research to fully elucidate its therapeutic potential for clinical implementation.
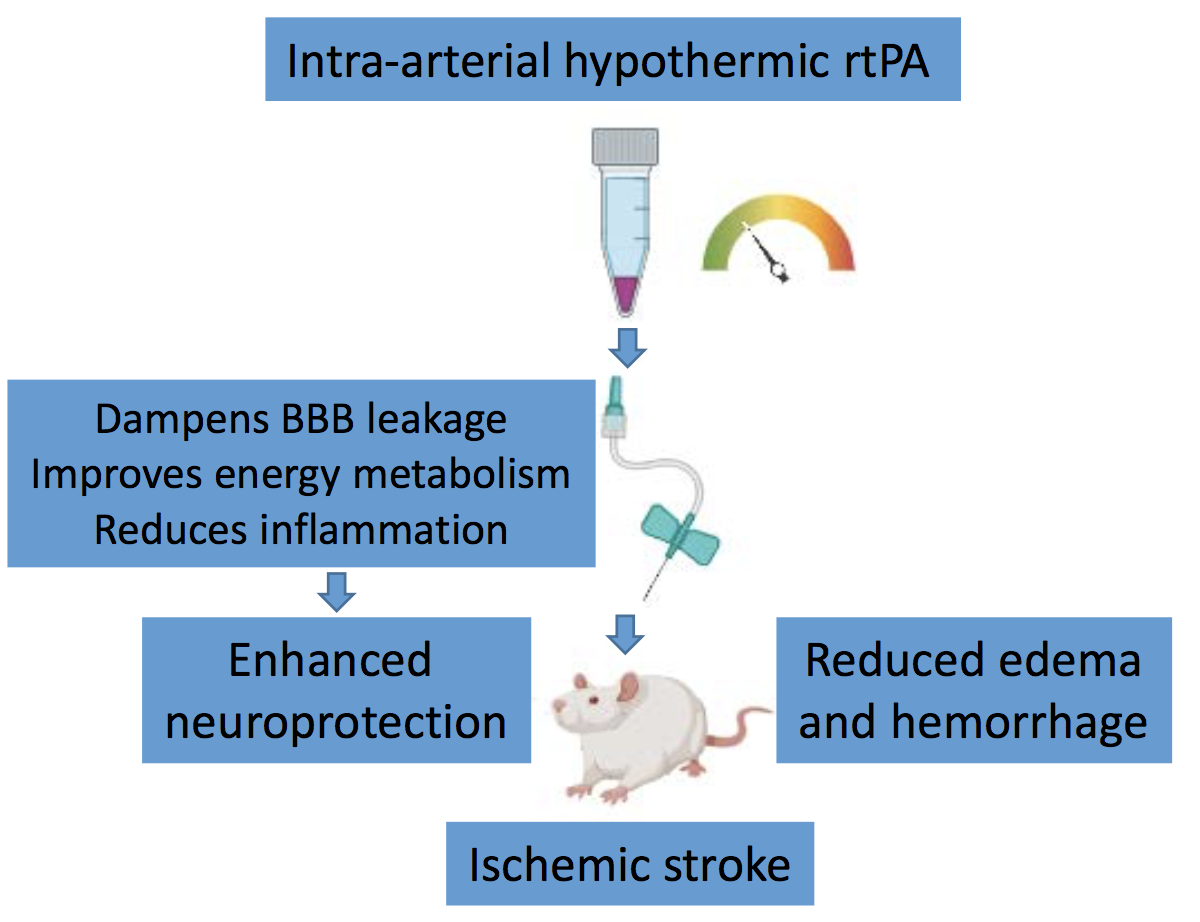
In a new window | Download PPT
Figure 1. Intra-arterial hypothermic rtPA for ischemic stroke. Adult rats subjected to experimental stroke received 2 mL of 4°C rtPA (0.07 mg/ml) via a modified PE-10 catheter (0.2-mm outer diameter and 0.1-mm inner diameter) inserted through an incision in the carotid artery to induce brain-specific hypothermia as soon as reperfusion began. This combination of selective hypothermia and thrombolytic rtPA reduces neurological deficits, cerebral infarction, and hemorrhagic transformation in stroke rats, possibly via multiple neuroprotective and neuroregenerative mechanisms, including dampening blood-brain barrier leakage, improving energy metabolism, and reducing inflammation.
Conflict of interest
We declare no conflict of interest.
References
Maximillian C. Borlongan1
1Vanderbilt University, Nashville, TN.
Mia C. Borlongan2
2California Northstate University, Rancho Cordova, CA.
Yumin Luo3,4,5*
3Institute of Cerebrovascular Diseases Research, Department of Radiology and Nuclear Medicine, Xuanwu Hospital of Capital Medical University, Beijing, China. 4Beijing Key Laboratory of Translational Medicine for Cerebrovascular Diseases, Beijing, China. 5Beijing Institute for Brain Disorders, Beijing, China.
Corresponding author:
Yumin Luo MD, PhD
Email: yumin111@ccmu.edu.cn

In a new window | Download PPT
Figure 1. Intra-arterial hypothermic rtPA for ischemic stroke. Adult rats subjected to experimental stroke received 2 mL of 4°C rtPA (0.07 mg/ml) via a modified PE-10 catheter (0.2-mm outer diameter and 0.1-mm inner diameter) inserted through an incision in the carotid artery to induce brain-specific hypothermia as soon as reperfusion began. This combination of selective hypothermia and thrombolytic rtPA reduces neurological deficits, cerebral infarction, and hemorrhagic transformation in stroke rats, possibly via multiple neuroprotective and neuroregenerative mechanisms, including dampening blood-brain barrier leakage, improving energy metabolism, and reducing inflammation.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 4784 | 4 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA