Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Stem cell-derived extracellular vesicles participate in the remodeling of stroke brain
Time:2024-05-09
Number:5663
Mauricio Muleiro Alvarez1, Felipe Esparza Salazar1, Maria Fernanda Osorio Martinez1, Francesco D’Egidio1, Thomas Rodriguez1, Jea-Young Lee1
Author Affiliations


- 1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Conditioning Medicine 2023. 6(5): 155-159.
Abstract
Strokes are among the diseases with the highest mortality and morbidity worldwide. Despite this, current stroke treatments have several limitations, prompting the exploration of new therapeutic options to address the post-acute phase. A key innovation involves the transplantation of stem cells, which exert their effects through various mechanisms. Notably, they release extracellular vesicles that contain anti-inflammatory factors, thereby reducing cellular damage and promoting neurogenesis. While the use of stem cells and extracellular vesicles is still under investigation, this review highlights that the utilization of extracellular vesicles presents several advantages. These advantages suggest that extracellular vesicles could potentially be a superior treatment option for recovering cognitive and motor function.
Keywords: Stroke, Stem cells, Exosome, Extracellular vesicles, Neurogenesis
Abstract
Strokes are among the diseases with the highest mortality and morbidity worldwide. Despite this, current stroke treatments have several limitations, prompting the exploration of new therapeutic options to address the post-acute phase. A key innovation involves the transplantation of stem cells, which exert their effects through various mechanisms. Notably, they release extracellular vesicles that contain anti-inflammatory factors, thereby reducing cellular damage and promoting neurogenesis. While the use of stem cells and extracellular vesicles is still under investigation, this review highlights that the utilization of extracellular vesicles presents several advantages. These advantages suggest that extracellular vesicles could potentially be a superior treatment option for recovering cognitive and motor function.
Keywords: Stroke, Stem cells, Exosome, Extracellular vesicles, Neurogenesis
Highlights
Successful treatment for stroke remains a significant unmet clinical need due to the narrow therapeutic window of current treatments targeting only the primary ischemic injury. Finding a treatment strategy that has a wider therapeutic window, especially sequestering the secondary cell death, will likely improve stroke outcomes. Here, we review the current status of stem cell therapy with emphasis on stem cell-derived extracellular vesicles, which appear to mitigate progressive secondary cell death by targeting inflammatory, angiogenic, vasculogenic, and neurogenic pathways. This extracellular vesicle-mediated brain remodeling process recapitulates conditioning medicine in that the treatment strategy facilitates the host neurovascular unit to mount a regenerative process against stroke.
Introduction
Strokes are one of the primary contributors to global mortality and long-term disability worldwide (Tirandi et al., 2023). In the United States alone, approximately 796,000 new cases are reported yearly, with 50% of those affected experiencing reduced mobility (Katan et al., 2019). Ischemic stroke, which causes about 70% of strokes, remains the most prevalent form (Feigin et al., 2017). Unfortunately, current treatments for acute ischemic stroke, such as endovascular treatment or intravenous thrombolysis with tissue plasminogen activator (tPA) (de Leciñana et al., 2014), only benefit a small number of ischemic stroke patients. Because of the short 4.5-hour therapeutic window, approximately 90% of ischemic stroke patients are not eligible for current treatments (Grossman et al., 2013; Knecht et al., 2018; Saceleanu et al., 2023). Therefore, finding new effective therapeutic strategies after the acute phase of ischemic stroke is essential to reduce mortality and enhance functional recovery in stroke survivors.
Stem cell therapy for stroke
Over the last few decades, researchers have been investigating a promising therapeutic strategy involving stem cells (SCs). These SCs have shown the potential to extend the treatment window even days after an ischemic event (Huang et al., 2019; Zhang et al., 2020). Animal studies have provided substantial evidence of the benefits of SC, as they can improve neurological function and reduce the size of the infarct (Borlongan et al., 2019; Chen et al., 2019; Huang et al., 2019; Zhang et al., 2021).
In both experimental and clinical studies, various types of SCs have been used as treatments. These include hematopoietic stem cells, embryonic stem cells, mesenchymal stem cells (MSC), neural stem cells, induced pluripotent stem cells, endothelial progenitor cells, and adult tissue-derived stem cells (Liu et al., 2009; Incontri Abraham et al., 2019; Custodia et al., 2022). Among them, MSC and neural stem cells have demonstrated significant effectiveness in promoting neuroprotection, immune regulation, astrogenesis, oligodendrogenesis, synaptogenesis, and improvements in motor and cognitive functions. They also play a role in secreting neurotrophic factors and growth factors and in remodeling the extracellular matrix (Zhang et al., 2020; Napoli et al., 2016; Napoli et al., 2018; Nguyen et al., 2019; Haghighitalab et al., 2021; Dabrowska et al., 2019; Regenhardt et al., 2020; Tuazon et al., 2019).
The use of SCs to treat stroke survivors is becoming increasingly prevalent in clinical studies (Lee et al., 2022; ( Chiu et al., 2022 ); de Celis-Ruiz et al., 2021). However, the effective production and ethical acquisition of sufficient SC are vital considerations when moving SC products from the laboratory to the clinical setting. Furthermore, identifying the most suitable cell type for exogenous SC transplantation requires careful evaluation of the effectiveness, accessibility, and ethical considerations (Incontri Abraham et al., 2019; Chase et al., 2021).
There are several proposed mechanisms by which SCs exert their effects. Two of the most well-documented mechanisms involve SCs replacing damaged cells and secreting paracrine factors, also known as the bystander effect. Transplanted neural stem cells and MSC can produce growth factors, such as vascular endothelial growth factor (VEGF), brain-derived neurotrophic factor (BDNF), and epidermal growth factor (EGF) (Huang et al., 2019). These substances can reduce apoptosis, oxidative stress, and neuroinflammation while also promoting mitochondrial repair, angiogenesis, vasculogenesis, and neurogenesis (Borlongan et al., 2004; Doeppner et al., 2010; Islam et al., 2021) (Figure 1).
Interestingly, the bystander effect’s mechanism suggests that when SCs are transplanted into the brains of stroke animals, they may not engraft well. Nevertheless, they still contribute to functional recovery, indicating that long-term survival and differentiation may not be necessary to repair the stroke-affected brain and restore its functions (Borlongan et al., 2004; Doeppner et al., 2010; Islam et al., 2021). This discovery challenges the notion that stem cell engraftment is required for brain repair, highlighting the importance of the therapeutic effects derived from the SC secretome. The secretome comprises extracellular vesicles (EVs) and exosomes that carry growth factors such as chemokines, cytokines, microRNA (miRNA), long noncoding RNA, and VEGF (Castelli et al., 2021; Kishida et al., 2019; Cunningham et al., 2018). Despite not fully understanding the precise mechanisms behind SC therapy, whether it involves cell replacement, bystander effects, or exosomal therapeutic effects, transplanting SCs into stroke-afflicted animals has shown significant reductions in secondary cell death, particularly in neuroinflammation, along with improved behavioral outcomes (Venkat el at., 2020; Zhang et al., 2019).
Neurorepair via stem cell-derived EVs
EVs are a set of membrane-bound structures classified into three subtypes: apoptotic bodies (50-4000nm), microvesicles (100-1000nm), and exosomes (30-150nm) (van Niel et al., 2018; Kalluri et al., 2020). Apoptotic bodies are released from cells undergoing apoptosis, microvesicles are released from the plasma membrane through clathrin-mediated shedding, and exosomes facilitate intercellular communication. EVs can contain different materials such as lipids, proteins, or genetic material, and they are found in almost all biological fluids, such as cerebrospinal fluid, plasma, saliva, urine, and serum (Valadi et al., 2007; van Niel et al., 2018). Moreover, EVs can carry functional organelles to help impaired cells in local and distant environments. In particular, mitochondria transferred from therapeutic cells via EVs showed promising results in improving the conditions of lesioned cells (Hayakawa et al., 2018; Rim et al., 2018). EVs are important in intercellular communication and are involved in pathological and physiological functions since they contribute to the progression and development of different inflammatory, autoimmune, metabolic, cancer metastasis, liver, and neurodegenerative and neuroinflammatory diseases (Izquierdo-Altarejos et al., 2024). Through extensive studies, MSC shows great promise for cell therapy and tissue repair, but MSC-derived EVs have several advantages compared to cell therapy: better safety, targeting, and versatility. MSC-derived EVs are less immunogenic and lack self-replicative capacity, thereby having no potential for tumor growth. EVs from MSCs help decrease neuroinflammation secondary to stroke. In animal models, the administration of MSC-derived EVs promotes neurogenesis, angiogenesis, and neural remodeling. EVs are much smaller than cells, allowing EVs greater mobility to target organs or tissues, like crossing the blood-brain barrier. EVs are modifiable to increase their therapeutic potential, as seen by a phase II clinical trial examining the efficacy of miR-124-enriched MSC-derived EVs on neurovascular remodeling and functional recovery after acute ischemic stroke (ClinicalTrials.gov: NCT03384433) (Izquierdo-Altarejos et al., 2024).
During a stroke, EVs are released into the blood, including neural cells, endothelial cells, progenitor cells, leukocytes, and platelets. They also possess brain-derived surface markers that include cell adhesion molecule L1, GPI-anchored prion protein, neural cell adhesion molecule, glutamate receptors 2 and 3, contactin-2, and proteins such as the glutamate chain light neurofilaments, neuron-specific enolase, and β-tubulin. Simak et al. (2006) reported increased levels of endothelial EVs during acute stroke compared to controls (Stenz et al., 2020; Ollen-Bittle et al., 2022). By their way of formation, EVs can be divided into exomes and microvesicles. Exosomes are small electrical vesicles with a diameter of 30 to 150 nm. They are formed from internal germination by an endosomal pathway to mature and become a multivesicular body to function with the plasma membrane. On the other hand, microvesicles are irregular in shape, measure from 50 to 1000 nm, and are produced by direct germination of the plasma membrane (Ollen-Bittle et al., 2022).
After the stroke, endothelial cells undergo an inflammatory process that promotes the release of EVs. Such EVs express endothelial cell antigens like vascular endothelium, endogin, phosphatidylserine, and cadherin, promoting a more inflammatory profile. Indeed, after exposure to inflammatory cytokines, vascular cells upregulate intracellular adhesion molecule 1 (ICAM-1). In addition, neurons can release EVs that contain miRNA-98, which helps to decrease microglial phagocytosis of neurons (Stenz et al., 2020; Ollen-Bittle et al., 2022).
With MSC-derived EVs showing great promise over cell therapy, extensive studies have been done to characterize the contents of these EVs. Many exosomal miRNAs that improve ischemia-induced brain damage were identified within the last few years. These exosomal miRNAs aid neurogenesis and have anti-inflammatory, immunomodulatory, and angiogenic effects. Exosomal miR-126 derived from adipose-derived (AD) MSCs and miR-184 derived from bone marrow-derived mesenchymal stem cells (BM-MSCs) induce behavioral recovery by promoting neurogenesis in stroke models (Xu et al., 2020; Gregorius et al., 2021; Fattahi et al., 2023). Exosomal miR-30d derived from AD-MSCs decreases brain infarcts by downregulating autophagy and enhancing M2 microglia polarization. Additionally, exosomal miR-138 derived from BM-MSCs inhibits astrocytes' inflammatory response, improving neurological function. Exosomal miR-29b derived from BM-MSCs enhances angiogenesis and inhibits neuronal apoptosis, alleviating ischemic brain injury. Exosomal miR-132 derived from BM-MSCs protects against ischemic stroke by dampening vascular oxidative stress and limiting blood-brain barrier damage (Nakano et al., 2021).
Recognizing the mechanisms that mediate EV therapeutic effects on stroke may provide a deeper understanding of their crucial role in abrogating the disease pathology. A study by Xin et al. (2022) utilized BV-2 microglial cells to uncover the mechanism behind the therapeutic action of MSC-derived EVs against hypoxia-ischemia (HI). After treating the BV-2 cells with MSC-derived EVs, Xin et al. (2022) found that the cells had increased viability and reduced expression of pro-inflammatory cytokines. Through further study, they found that MSC-derived EVs alleviate HI-induced brain injury in neonatal mice by transporting miR-21a-5p, promoting the protective M2 polarization of microglial cells by arresting signal transducer and activator of transcription 3 (Xin et al., 2022). Additionally, a study by Liu et al. (2023) established an ischemic brain injury mouse model to understand the mechanism behind the potential therapeutic effects of MSC-derived EVs on ischemic stroke. After injection of MSC-derived EVs, they noted increased interleukin-33 (IL-33) with decreased tumorigenicity 2 receptor (ST2) in the ischemic penumbra, resulting in functional recovery. They concluded that the MSC-derived EVs decrease the volume of cerebral infarction by inhibiting oxygen and glucose deprivation-induced cell death via an astrocytic IL-33/ST2 signaling mechanism (Liu et al., 2023).
Our recent study similarly demonstrates the participation of SC-derived EVs in functional recovery in an animal model of stroke (Lee et al., 2024). Using a population of non-adherent CD34+ cells derived from human peripheral blood called novel ProtheraCytes®, which have been processed under good manufacturing practice and have been tested in a Phase 2 clinical trial in post-acute myocardial infarction (NCT02669810), we show that ProtheraCytes® secrete EVs that promote angiogenesis and vasculogenesis. In this study, intranasal transplantation of ProtheraCytes® three days after experimentally induced stroke (via transient middle cerebral artery occlusion) in adult rats ameliorated stroke-induced behavioral dysfunctions and ischemic injury at about a month after stroke (Lee et al., 2024). These functional outcomes indicate that minimally invasive intranasal delivery may be a practical and effective approach for clinical transplantation of stem cells. Equally important, human CD63+ EVs were elevated in the ischemic brains of stroke animals that received ProtheraCytes®, coupled with enhanced angiogenic and vasculogenic markers, dampened inflammation, and increased neurogenesis (Lee et al., 2024). These observations advance the utility of clinical grade ProtheraCytes® as a stroke therapy and highlight the implications of the EV-induced brain remodeling process. In tandem, these findings provide clinical insights into the design of intranasal cell therapy in stroke using EVs as a sensitive biomarker for monitoring the functional response of patients to the transplanted SCs.
Conclusion
Current stroke treatment focuses on preventing the progress of the occlusion or embolism, thus improving symptoms and prognosis. However, none of the existing therapies focus on improving the damage caused by secondary cell death. In this mini-review, we found that stem cells and their EVs serve as a robust treatment strategy in preventing this secondary cell death by engaging the inflammatory, angiogenic, vasculogenic, and neurogenic processes during the progressive stage of stroke. Thus, in the future, researchers need to deepen the knowledge around EVs’ mechanisms, physiologically and pathologically, after cell therapy to better depict their involvement in stroke and their ability to improve brain repair. Additional studies are warranted to further probe the safety and efficacy of EV-based therapies before they can be considered as a viable option to improve the quality of life of stroke patients.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
Mauricio Muleiro Alvarez1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Felipe Esparza Salazar1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Maria Fernanda Osorio Martinez1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Francesco D’Egidio1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Thomas Rodriguez1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Jea-Young Lee1
1Center of Excellence for Aging and Brain Repair, Department of Neurosurgery and Brain Repair, Morsani College of Medicine, University of South Florida, 12901 Bruce B. Downs Blvd., Tampa, FL 33612, USA.
Corresponding author:
Jea-Young Lee
Email: jeayoung@usf.edu
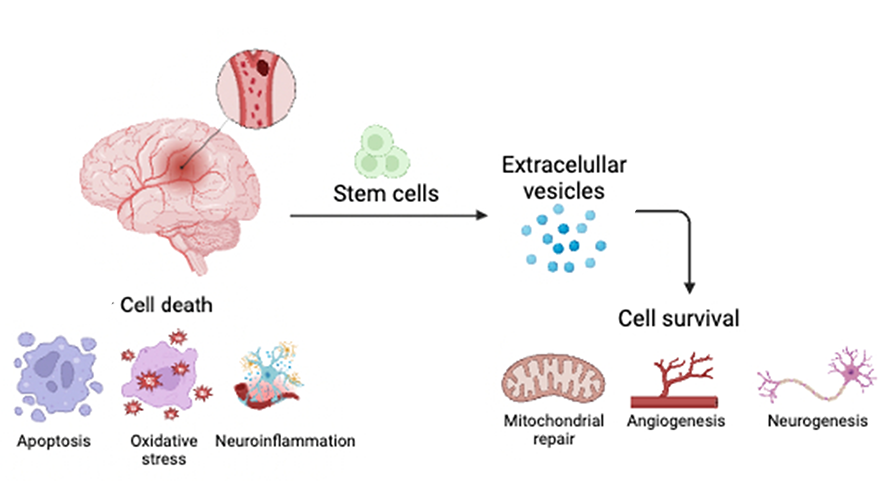
In a new window | Download PPT
Figure 1. After a stroke, a cascade of cell death events ensues, leading to progressive secondary cell death. The use of stem cells may sequester these secondary cell death processes by releasing extracellular vesicles, aiding in brain remodeling and functional recovery.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5663 | 17 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA