Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Restoring mesocortical dopamine neurotransmission to improve obesity-induced cognitive dysfunction
Time:2024-09-17
Number:5704
Ike de la Peña1, Thomas Rodriguez2, Shant Krikorian1, Peter Haydaw1
Author Affiliations


- 1Department of Pharmaceutical and Administrative Sciences, Loma Linda University School of Pharmacy, Loma Linda California, USA.
- 2Loma Linda University School of Medicine, Loma Linda, California, USA.
Conditioning Medicine 2023. 6(6): 170-176.
Abstract
Obesity presents a significant clinical burden, increasing the risk of various serious health complications and diseases. Recently, cognitive impairment has emerged as an important obesity-associated pathology. Several lines of evidence suggest that obesity impacts the mesocortical dopamine pathway, which plays a crucial role in cognition and higher-order executive functions. Furthermore, there are structural and functional alterations in the prefrontal cortex (PFC) and its associated networks, highlighting the involvement of the PFC in this pathological state. Therefore, interventions that restore the mesocortical pathway and enhance PFC function may offer promising avenues for addressing obesity-induced cognitive dysfunction. In this paper, we discuss strategies encompassing both pharmacological and non-pharmacological interventions to ameliorate obesity-induced cognitive dysfunction. Pharmacological treatments targeting dopamine neurotransmission are presented, either as standalone therapies or in combination with other drugs, to synergistically ameliorate cognitive dysfunction. Non-pharmacological approaches such as brain stimulation therapies and exercise are also discussed as viable options for this condition. While various approaches could address obesity-induced cognitive deficits, focusing on the mesocortical pathway and the PFC represents rational interventions that could enhance cognitive control, thereby facilitating sustainable weight loss and further improving cognitive function in the long term.
Keywords: obesity, cognitive dysfunction, drugs, combination treatment, brain stimulation
Abstract
Obesity presents a significant clinical burden, increasing the risk of various serious health complications and diseases. Recently, cognitive impairment has emerged as an important obesity-associated pathology. Several lines of evidence suggest that obesity impacts the mesocortical dopamine pathway, which plays a crucial role in cognition and higher-order executive functions. Furthermore, there are structural and functional alterations in the prefrontal cortex (PFC) and its associated networks, highlighting the involvement of the PFC in this pathological state. Therefore, interventions that restore the mesocortical pathway and enhance PFC function may offer promising avenues for addressing obesity-induced cognitive dysfunction. In this paper, we discuss strategies encompassing both pharmacological and non-pharmacological interventions to ameliorate obesity-induced cognitive dysfunction. Pharmacological treatments targeting dopamine neurotransmission are presented, either as standalone therapies or in combination with other drugs, to synergistically ameliorate cognitive dysfunction. Non-pharmacological approaches such as brain stimulation therapies and exercise are also discussed as viable options for this condition. While various approaches could address obesity-induced cognitive deficits, focusing on the mesocortical pathway and the PFC represents rational interventions that could enhance cognitive control, thereby facilitating sustainable weight loss and further improving cognitive function in the long term.
Keywords: obesity, cognitive dysfunction, drugs, combination treatment, brain stimulation
Highlights
Obesity-induced cognitive dysfunction is an important emerging neurological consequence of obesity, for which no known treatments specifically addressing this medical condition have ever been developed. The mesocortical dopamine pathway, emanating from the ventral tegmental area to the prefrontal cortex (PFC), which modulates a range of cognitive processes, is known to be involved in this pathological condition. This review manuscript discusses strategies addressing obesity-induced cognitive dysfunction, focusing on treatments that enhance mesocortical neurotransmission and improve dopamine levels in the PFC. Such treatments may represent a novel area of conditioning medicine that can curtail the cognitive deficits associated with obesity.
Introduction
Obesity has evolved into a global pandemic, exerting a significant toll on individuals' overall physical, emotional, and mental well-being (Luppino et al., 2010; The Lancet Gastroenterology and Hepatology, 2021). One important emerging consequence of obesity that has garnered substantial attention in recent years is cognitive dysfunction or impairment (Bocarsly et al., 2015; O'Brien et al., 2017; Salas-Venegas et al., 2022). Evidence indicates that obesity negatively impacts the central nervous system, particularly cognitive functions such as attention, executive function, and decision-making (O'Brien et al., 2017). Indeed, systematic reviews and meta-analyses have consistently shown a strong association between measures of central obesity, such as high waist circumference, and heightened risks of cognitive impairment and dementia (Tang et al., 2021). Longitudinal cohort studies have also revealed a higher risk of dementia development among individuals with a high body mass index (BMI) in mid-life (Qu et al., 2020).
Of relevance to obesity-induced cognitive dysfunction are key brain regions implicated in cognition, notably the hippocampus and prefrontal cortex (PFC). Structural alterations, including reductions in hippocampal grey matter volume and compromised white matter integrity, have been consistently observed in obese cohorts, potentially affecting cognitive and memory functions (Ward et al., 2005; Raji et al., 2010; Bolzenius et al., 2015). Similarly, neuroimaging studies have revealed grey matter atrophy in the parietal, temporal, and frontal lobes, particularly affecting the PFC and its executive function capacities (Fernández-Andújar et al., 2021). Proposed mechanisms underlying these alterations include low-grade systemic inflammation, blood-brain barrier disruption, central inflammation, microglial activation, and induction of pro-inflammatory proteins, resulting in neuronal demise, impaired neurogenesis, and synaptic remodeling, especially in the hippocampus and the PFC (Solas et al., 2017; de la Peña et al., 2023). Notably, the interactions between the hippocampus and PFC have been proposed as playing a key role in various cognitive and behavioral functions (for review, see Ruggiero et al., 2021). Therefore, obesity and/or diet-induced structural alterations in both PFC and hippocampus may affect hippocampal-PFC function and connectivity and contribute to the development of cognitive deficits in obese individuals.
Neurotransmitter systems, such as the cholinergic, glutamatergic, and dopaminergic systems, are important for cognitive processes, including learning and memory. Notably, reduced dopamine levels have been consistently documented in both obese humans and animal models (Geiger et al., 2008; Nguyen et al., 2017; Lowe et al., 2019), with evidence suggesting that consumption of obesogenic diets disrupts the brain's dopamine network (Wang et al., 2001; Barry et al., 2018), thereby impairing learning and memory functions. This highlights the critical role of dopamine in obesity-induced cognitive deficits (de la Peña et al., 2022).
Prevention and treatment strategies can potentially alleviate obesity-induced cognitive dysfunction. Moreover, interventions that improve cognitive impairment may promote better dietary self-regulation, encouraging healthier food choices and sustainable weight management in obese individuals (Lowe et al., 2019; de la Peña et al., 2022; Devoto et al., 2023). Despite progress in understanding the mechanisms behind obesity-induced cognitive dysfunction, specific treatments addressing the cognitive consequences of obesity have not yet been established. In this article, we discuss the role of the mesocortical dopamine pathway in the pathology of obesity-induced cognitive dysfunction and strategies targeting this pathway and the PFC to address the cognitive deficits associated with obesity.
The mesocortical pathway in obesity-induced cognitive dysfunction
Distinct dopaminergic pathways mediate specific physiological functions. Among these, the mesocortical dopamine pathway, originating from the ventral tegmental area (VTA) and projecting to PFC, plays a crucial role in modulating cognition and higher-order (i.e., executive) cognitive processes (McClure et al., 2004; Nobili et al., 2017; Coenen et al., 2018). These processes include resolving new tasks, modifying existing behaviors, strategic planning for problem-solving, and regulating thoughts and actions during goal-directed behavior (Diamond, 2013). The executive system also controls other cognitive processes, including memory, attention, and reasoning (Diamond, 2013). Besides these functions, the mesocortical pathway, in coordination with other dopamine pathways (e.g., mesolimbic), also regulates emotions and motivated behavior. Thus, it is widely recognized to play a crucial role in the pathophysiology of several neuropsychiatric disorders, such as schizophrenia, attention-deficit/hyperactivity disorder, depression, and bipolar disorders (Ayano, 2016; Li et al., 2016).
Numerous cross-sectional studies consistently indicate that individuals with obesity show poorer performance across multiple cognitive domains, particularly executive functioning, throughout their lifespan (Hayes et al., 2018; Favieri et al., 2019). These effects persist regardless of age, gender, or comorbid health conditions known to impact executive functioning, such as cerebrovascular disease, hypertension, and type 2 diabetes mellitus (Gunstad et al., 2007; Yang et al., 2018). Collectively, the above evidence indicates the significant impact of obesity on the mesocortical dopamine pathway.
Moreover, animal studies have demonstrated that exposure to a high-fat diet induces substantial cognitive impairment in rodents (Kothari et al., 2017; Liang et al., 2023), suggesting that dietary factors may directly affect cognitive functioning. The exact mechanisms by which obesity and/or an obesogenic diet produce these effects are not fully understood. However, neurophysiological and metabolic factors have been implicated, such as changes in insulin sensitivity, inflammatory processes stemming from body fat accumulation, and alterations in cerebrovascular blood flow (Solas et al., 2017). These factors, whether acting in concert or independently, could precipitate structural changes in the mesocortical pathway, resulting in functional alterations, particularly in the PFC, and consequently, deficits in executive functioning (Lowe et al., 2019). Indeed, animal studies have shown that exposure to an obesogenic diet disrupts dopaminergic brain networks, including the mesocortical pathway (Barry et al., 2018), corroborating the aforementioned hypothesis. Nevertheless, environmental factors, such as early-life adversity (Reichelt, 2016), which is known to profoundly impact the mesocortical pathway, may also influence this effect.
Prefrontal cortex dysfunction in obesity
Several reviews have provided an extensive summary of the role of the PFC and the impact of obesity on the PFC (Smith and Robbins, 2013); Favieri et al., 2019; Lowe et al., 2019). Neuroimaging studies have further revealed PFC dysfunction in obesity (for review, see Lowe et al., 2019). For instance, these studies consistently showed smaller whole brain volumes and reduced regional grey matter volumes in various brain regions among individuals with obesity compared to those of healthy weight. These regions include the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex, ventromedial PFC, and frontopolar cortex. Moreover, obesity correlates with grey matter atrophy in the frontal lobes, with frontal atrophy observed across different age groups. Specifically, children and adolescents with obesity have reduced grey matter volume, most prominently in the frontal and limbic regions. Given that these obesity-related differences in brain morphometry are linked to impairments in executive functions, alterations in grey matter morphometry may mediate the relationship between obesity and cognitive functioning (Lowe et al., 2019).
Furthermore, significant reductions in PFC dopamine levels have been observed in both obese humans and animals (Geiger et al., 2008; Nguyen et al., 2017; Lowe et al., 2019), indicating the critical role of dopamine in obesity-related cognitive deficits and dysfunctional eating behavior. Consequently, treatments that restore mesocortical dopaminergic neurotransmission, thereby enhancing dopamine levels in the PFC (Lowe et al., 2019; de la Peña et al., 2022; Devoto et al., 2023), may ameliorate obesity-associated cognitive dysfunction (Figure 1). Moreover, considering the PFC's role in modulating food intake, such treatments could potentially facilitate sustainable weight loss by enhancing cognitive control and executive functions. Cognitive control refers to the ability to regulate and manage cognitive processes to achieve specific objectives, encompassing functions such as inhibitory control, working memory, cognitive flexibility, and attentional control (Ott and Nieder, 2019; Friedman and Robbins, 2022). Several studies have established a link between cognitive control and cognitive performance, particularly in terms of executive function and overall cognitive proficiency (Ott and Nieder, 2019; Friedman and Robbins, 2022). Notably, cognitive functions are suggested to be heavily influenced by dopamine, and that dopamine facilitates successful cognitive control in the PFC (Ott and Nieder, 2019).
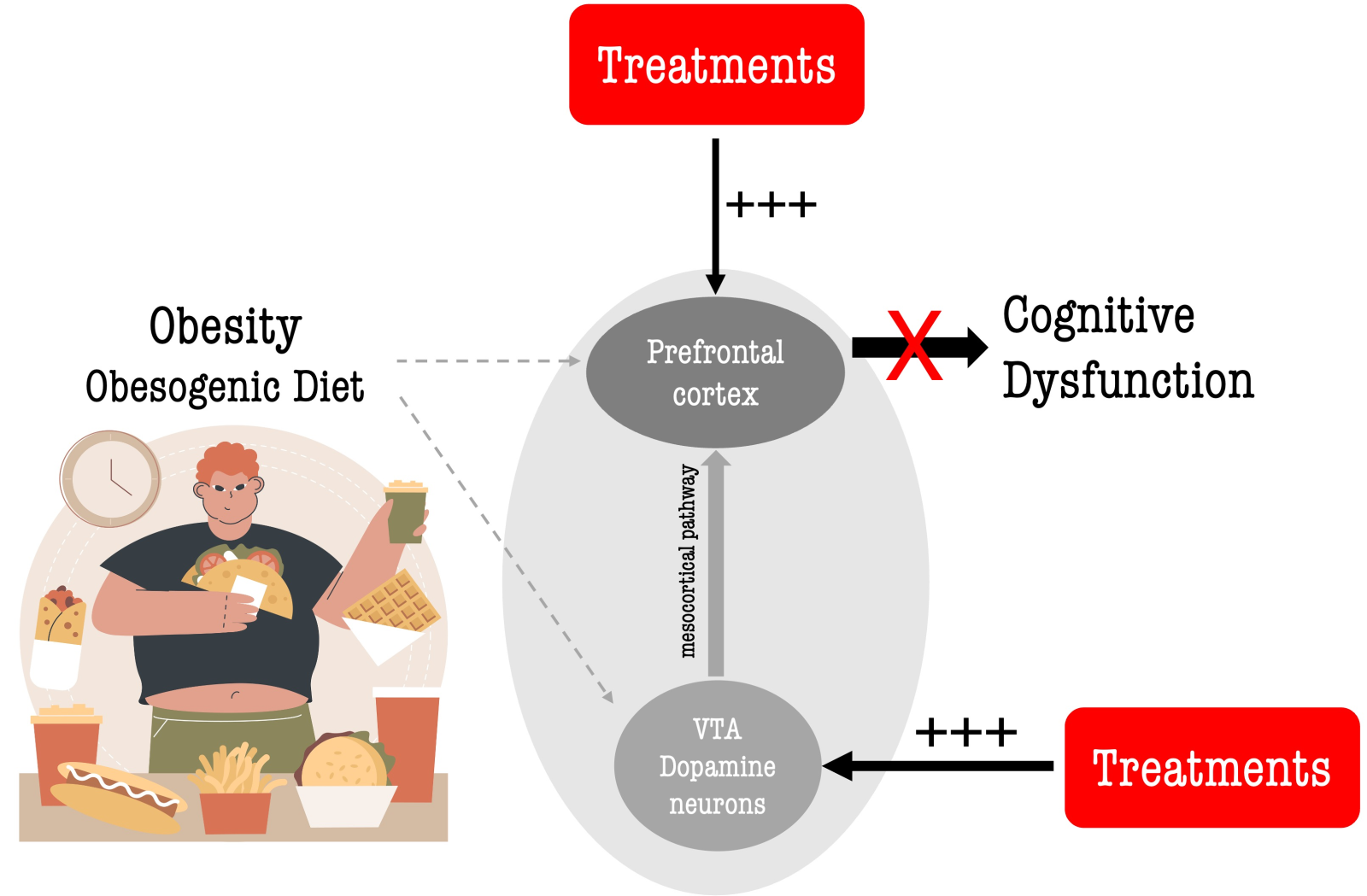
In a new window | Download PPT
Figure 1. Restoring mesocortical dopamine neurotransmission to ameliorate obesity-induced cognitive dysfunction. Obesity and/or obesogenic diets (e.g., high fat) negatively impact cognitive functions through numerous mechanisms (for review, see de la Pena et al., 2023). Evidence suggests that obesity and/or obesogenic diets disrupt dopaminergic brain networks, including the mesocortical dopamine pathway originating from the ventral tegmental area (VTA) to the prefrontal cortex (PFC), which is involved in regulating cognition and higher-order cognitive processes. This alteration results in poorer performance in various cognitive domains in obese individuals. Restoring the mesocortical pathway through interventions (pharmacological and non-pharmacological) may enhance dopamine neurotransmission and/or PFC function and ameliorate obesity-induced cognitive deficits. Image created by Freepik.
Strategies to Restore Mesocortical Dopamine Signaling to Ameliorate Obesity-Associated Cognitive Dysfunction
In the sections below, we discuss probable strategies to improve obesity-induced cognitive dysfunction. It is worth noting that weight loss may also ensue as a concurrent outcome of these interventions, owing to diverse mechanisms such as enhancing dopamine levels and/or dopaminergic neurotransmission (Simonds and Cowley, 2019).
Pharmacological treatments
Recently, pharmacotherapy for obesity has been improved with new and more effective centrally acting drugs (Chakhtoura et al., 2023; Coutinho and Halpern, 2024). Pharmacotherapy can also be considered to alleviate cognitive dysfunction in obese individuals. Psychostimulants (e.g., amphetamine and phentermine) increase brain dopamine levels through various mechanisms (e.g., dopamine reuptake inhibition, promoting dopamine release) and are widely recognized for their capacity to induce weight loss (Poulton et al., 2016; Stemmer et al., 2019). Consequently, some stimulants have obtained approval as weight-loss medications, such as phentermine (Adipex-P, ProFast), benzphetamine (Didrex™, a substituted amphetamine), and diethylpropion (Tenuate™). Moreover, some evidence suggests cognitive-enhancing effects of psychostimulants. For example, amphetamine has been reported to improve consolidation and recall, as well as to enhance executive function and attention (Advokat, 2010; Bagot and Kaminer, 2014). However, long-term use of psychostimulants has been associated with several reported adverse effects (Stemmer et al., 2019). Amphetamine, for instance, has been linked to various neurotoxic effects, including degradation of synaptic terminals and neuronal chromatolysis in the cortex and striatum, as well as permanent loss of dopamine uptake sites in the striatum and nucleus accumbens (Robinson and Kolb, 2004; Urban and Gao, 2017). It can also induce a schizophrenia-like psychosis characterized by hallucinations, paranoia, panic, and hyperactivity (Urban and Gao, 2017). A case report has further documented recurring psychotic episodes with phentermine administration (Jo et al., 2019). Combined with topiramate, phentermine has been reported to cause memory impairment (Hainer and Aldhoon-Hainerová, 2014). Furthermore, the ability of these drugs to elevate dopamine levels, particularly in the nucleus accumbens, raises concerns about their potential for abuse (de la Peña et al., 2015). While there is generally no established association between obesity and substance abuse (Hu et al., 2020), findings from certain studies revealing high rates of substance abuse among obese individuals underscore the need for alternative drug treatments (Sansone and Sansone, 2013). Preferably, non-psychostimulant anti-obesity drugs that could enhance cognition should be identified to mitigate the risk of drug abuse among obese individuals.
Bupropion, a weight-loss medication combined with naltrexone, operates similarly to psychostimulants by inhibiting the reuptake of dopamine and norepinephrine (Guglielmi et al., 2023). While not categorized as a psychostimulant, it can normalize cognitive performance in patients with depression (Colwell et al., 2022). Bupropion-induced increases in dopamine neurotransmission in the PFC may produce cognitive improvement in obese individuals.
Levodopa (L-DOPA), a drug primarily used in the treatment of Parkinson’s disease, increases dopamine levels in the brain by serving as a precursor that is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase within dopaminergic neurons. L-DOPA enhances frontal-subcortical and posterior cortical cognitive functioning in patients with Parkinson's disease (Gul and Yousaf, 2019). It can also improve learning and memory deficits in a mouse model of Alzheimer's disease (Ambrée et al., 2009). Moreover, preclinical studies have indicated improved cognitive function following global cerebral ischemia/reperfusion injury in rodents (Wang et al., 2020). Given these findings, L-DOPA could also be examined for its potential cognitive-enhancing effects within the context of obesity.
Furthermore, non-stimulant medications with established efficacy in weight reduction and in enhancing brain dopamine levels could also undergo evaluation for their effect on obesity-induced cognitive dysfunction. Examples include lorcaserin, a serotonin 5-HT2C receptor agonist, and betahistine, a dual histamine 1 receptor agonist and histamine 3 receptor antagonist. These drugs have individually shown effects on dopaminergic signaling through various pathways, suggesting potential modulation of cognitive function and food intake directly and indirectly via dopamine neurotransmission (for review, see de la Peña et al., 2022). Animal studies have demonstrated cognitive-enhancing effects of lorcaserin in obese mice (Yang et al., 2015), while high doses of betahistine have been found to enhance working memory in healthy human subjects (van Ruitenbeek and Mehta, 2013).
Furthermore, a recently approved weight loss medication, the glucagon-like peptide-1 receptor agonist liraglutide, has been shown to modulate associative learning in individuals with obesity. While the precise mechanism underlying this effect remains incompletely understood, it has been suggested that liraglutide may improve learning by indirectly impacting dopamine function (Hanssen et al., 2023). However, as discussed below, there may be potential benefits in combining treatments with medications, given the profound dopamine reduction in the PFC and the multifactorial pathology in obesity.
Combination Treatment
The complex pathology of obesity indicates the potential efficacy of combination treatment strategies in promoting weight loss and addressing obesity-related outcomes (Apovian et al., 2015). Combining anti-obesity drugs with diverse pharmacodynamic actions, influencing multiple pathways, may help overcome the inherent compensatory mechanisms involved in energy homeostasis, thus enhancing their effectiveness (Gadde and Allison, 2009). Similarly, combining drugs that work synergistically or in parallel may offer substantial benefits in addressing obesity-related cognitive dysfunction.
In a previous report, we proposed the concept of a combination treatment involving lorcaserin and betahistine to mitigate obesity-induced cognitive dysfunction (de la Peña et al., 2022). Co-administration of these drugs could synergistically enhance mesocortical dopamine release through their individual effects on 5-HT2C or histamine 1 and 3 receptors (de la Peña et al., 2022). By restoring mesocortical dopaminergic signaling, the combined treatment might not only alleviate obesity-induced cognitive dysfunction but also bolster cognitive control over food intake. These effects could potentially be achieved with minimal side effects at low doses of each drug. Notably, as these are non-psychostimulants that do not increase dopamine levels in the striatum or nucleus accumbens, the risk of abuse associated with these treatments may be minimized (de la Peña et al., 2022).
It is important to acknowledge that while lorcaserin has demonstrated remarkable efficacy in reducing food intake and body weight in obesity clinical trials, it has been withdrawn from the market due to adverse effects such as valvular disorder and probable cancer occurrence (Gorelik et al., 2020; de Andrade Mesquita et al., 2021). However, recent advancements have led to the development of new positive allosteric modulators of the 5-HT2C receptor, which have shown promise in intensifying 5-HT action for reducing food intake and weight loss in select in vivo studies (Przegaliński et al., 2023). Moreover, these selective 5-HT2CR positive allosteric modulators exhibit minimal affinity for 5-HT2BR-linked cardiac valves (Przegaliński et al., 2023), offering a potentially safer pharmacological approach to obesity treatment. Furthermore, new histamine 3 receptor antagonists (Mika et al., 2021) have been developed that demonstrate efficacy in preventing weight gain in a rat model of excessive eating. Firstly, it is important to establish the efficacy and safety profiles of these newly developed drugs for treating obesity. Subsequently, trials should be designed to investigate whether these drugs could influence dopaminergic signaling and whether their combined use could synergistically enhance dopamine neurotransmission. Furthermore, future experiments should explore whether this combined approach could improve cognitive function in obese subjects.
Non-pharmacological Treatments
In addition to pharmacological treatments, non-pharmacological approaches, such as brain stimulation therapies and aerobic exercise, can also be utilized to address obesity-induced cognitive dysfunction. Brain stimulation therapies involve the application of controlled electrical impulses or magnetic fields to specific brain regions, modulating brain activity. These therapies are commonly used to manage various neurological and psychiatric conditions and can be categorized as non-invasive or invasive (Lee et al., 2018; Pleger, 2018). Non-invasive methods, including transcranial magnetic stimulation (TMS) variants such as deep TMS (dTMS) or repetitive TMS (rTMS), as well as transcranial direct current stimulation (tDCS), safely modulate brain activity without requiring neurosurgical intervention (Sun et al., 2023). Due to their limited penetration depth, both TMS and tDCS can effectively target superficially localized brain regions (Pleger, 2018). Furthermore, rTMS or tDCS applied to specific brain areas may influence activity in interconnected distant brain sites (Klomjai et al., 2015).
Emerging evidence suggests that non-invasive brain stimulation targeting the DLPFC may enhance inhibitory control capacities over automatic processes associated with food craving, reward valuation, and attentional biases toward high-caloric food (Sedgmond et al., 2019). A recent systematic review comprising 19 randomized controlled trials reported that non-invasive brain stimulation techniques reduced body weight and food cravings compared to sham stimulation (Alhindi et al., 2023). These findings not only highlight the potential of non-invasive brain stimulation as a tool for treating obesity but also suggest their potential to improve cognition, evidenced by strengthened cognitive control over food cravings. It is plausible that non-invasive brain stimulation targeting the PFC enhances dopamine activity in the striatum and VTA, thereby augmenting PFC dopamine levels (Devoto et al., 2023; Di Domenico and Mapelli, 2023). Despite these promising outcomes, further research is needed, particularly to evaluate the overall cognitive functions of obese individuals following non-invasive brain stimulation. In addition, extensive investigations are warranted to elucidate the biochemical mechanisms underlying brain stimulation.
Aerobic exercise and resistance training interventions have both demonstrated significant improvements in executive function (Gomez-Pinilla and Hillman, 2013; Mandolesi et al., 2018). Moreover, exercise has also been shown to protect against obesity-induced cognitive dysfunction in animal models (Park et al., 2019) and obese individuals (for review, see Bourbeau et al., 2023). These findings suggest the effectiveness of non-pharmacological approaches in addressing obesity-induced cognitive deficits. Importantly, exercise and resistance training may also enhance dopamine levels (Gorrell et al., 2022), which could contribute to cognitive as well as mood-enhancing effects.
As mentioned earlier, combination treatment strategies offer benefits in promoting weight loss and addressing obesity-related cognitive deficits. Therefore, exploring combinations of not only medications but also drug and non-drug treatments could prove beneficial for this condition. For instance, combining medications with physical exercise may synergistically increase PFC dopamine levels. Physical exercise can also be complemented by other non-pharmacological approaches, such as cognitive and dietary interventions, which have been shown in clinical studies to improve cognition and mitigate obesity-related complications (Keawtep et al., 2024). The anticipated safety and efficacy of the specific combination treatment should be carefully considered when deciding whether to use non-drug and pharmacological interventions or combinations of two non-pharmacological treatments to resolve obesity-induced cognitive dysfunction.
Concluding Remarks
Despite the compelling evidence linking obesity with cognitive dysfunction, there are currently no specific treatments developed to address this pathological condition. In this paper, we discussed both pharmacological and non-pharmacological interventions to mitigate obesity-induced cognitive deficits. However, thorough safety and efficacy assessments are required to determine the viability of these treatments. While we propose that the above therapies impact the mesocortical pathway, it is also plausible that they affect other dopaminergic pathways, such as the mesolimbic pathway, thereby potentially contributing to their therapeutic effects. Of note, dopaminergic inputs to the forebrain, including the ventral striatum, form neural circuits vital for various cognitive and executive functions, including working memory, problem-solving, and various forms of behavioral flexibility (Floresco and Magyar, 2006).
Considering the role of other neurotransmitters in cognition and obesity-induced cognitive dysfunction, it is essential to explore drug development targeting systems like acetylcholine, glutamate, and norepinephrine to alleviate obesity-induced cognitive deficits. Additionally, addressing other mechanisms implicated in obesity-induced cognitive deficits, such as neuroinflammation and insulin resistance, can contribute to the development of a comprehensive arsenal of treatments for this disorder. For instance, tirzepatide, a dual GLP-1 agonist and glucose-dependent insulinotropic polypeptide (GIP) receptor agonist, improves spatial learning and memory impairment in high-fat diet-exposed rats by modulating aberrant insulin resistance and inflammation (Guo et al., 2023). Notably, non-pharmacological approaches, including plants and isolated compounds, have also been reported to improve obesity-induced cognitive dysfunction in animal models by attenuating neuroinflammation, improving central and peripheral insulin resistance, and affecting other pathways (de la Peña et al., 2023). Nevertheless, targeting the mesocortical pathway, particularly the PFC, remains a rational strategy for addressing obesity-induced cognitive dysfunction. Focusing on the PFC may not only enhance cognition but also improve cognitive control, enabling obese individuals to make healthier lifestyle choices and better manage food cravings. This could potentially lead to sustainable weight loss and further enhancement of cognitive function over the long term.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This study was funded by the American Association of Colleges of Pharmacy (AACP) New Investigator Grant and the Loma Linda University School of Medicine GRASP Seed Funds.
References
Ike de la Peña1
1Department of Pharmaceutical and Administrative Sciences, Loma Linda University School of Pharmacy, Loma Linda California, USA.
Thomas Rodriguez2
2Loma Linda University School of Medicine, Loma Linda, California, USA.
Shant Krikorian1
1Department of Pharmaceutical and Administrative Sciences, Loma Linda University School of Pharmacy, Loma Linda California, USA.
Peter Haydaw1
1Department of Pharmaceutical and Administrative Sciences, Loma Linda University School of Pharmacy, Loma Linda California, USA.
Corresponding author:
Ike de la Peña, Ph.D.
Email: idelapena@llu.edu

In a new window | Download PPT
Figure 1. Restoring mesocortical dopamine neurotransmission to ameliorate obesity-induced cognitive dysfunction. Obesity and/or obesogenic diets (e.g., high fat) negatively impact cognitive functions through numerous mechanisms (for review, see de la Pena et al., 2023). Evidence suggests that obesity and/or obesogenic diets disrupt dopaminergic brain networks, including the mesocortical dopamine pathway originating from the ventral tegmental area (VTA) to the prefrontal cortex (PFC), which is involved in regulating cognition and higher-order cognitive processes. This alteration results in poorer performance in various cognitive domains in obese individuals. Restoring the mesocortical pathway through interventions (pharmacological and non-pharmacological) may enhance dopamine neurotransmission and/or PFC function and ameliorate obesity-induced cognitive deficits. Image created by Freepik.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 5704 | 11 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA