International bi-monthly journal of cell signaling, tissue protection, and translational research.
Epigenetic regulation of NCX1 and NCX3 genes in cerebral ischemia and ischemic brain preconditioning
Silvia Ruggiero1, Natascia Guida1, Luca Sanguigno1, Luigi Formisano1
Author Affiliations


- 1Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 8013, Naples, Italy.
Abstract
Ischemic preconditioning refers to a sub-lethal and non-injurious ischemic insult that can activate complex endogenous mechanisms to provide ischemic protection and tolerance against a subsequent, otherwise lethal, ischemic insult. Over the years, it has been demonstrated that the sodium-calcium exchanger (NCX) can play a significant role in cerebral ischemia and brain ischemic preconditioning. Several studies showed that the expression of NCX1 and NCX3 isoforms can be triggered by epigenetic modifications, consisting of changes in: (1) the acetylation levels of histone proteins or (2) DNA methylation on their promoter sequences. Identifying the epigenetic mechanisms related to NCX1 and NCX3-induced neuroprotection in brain ischemic preconditioning could aid in discovering new epigenetic drugs that, by altering DNA or chromatin structure of NCX1 and NCX3 gene promoters, might reduce the infarct volume after a harmful ischemic insult.
Keywords: NCX1, NCX3, Neuroprotection, Epigenetic
Abstract
Ischemic preconditioning refers to a sub-lethal and non-injurious ischemic insult that can activate complex endogenous mechanisms to provide ischemic protection and tolerance against a subsequent, otherwise lethal, ischemic insult. Over the years, it has been demonstrated that the sodium-calcium exchanger (NCX) can play a significant role in cerebral ischemia and brain ischemic preconditioning. Several studies showed that the expression of NCX1 and NCX3 isoforms can be triggered by epigenetic modifications, consisting of changes in: (1) the acetylation levels of histone proteins or (2) DNA methylation on their promoter sequences. Identifying the epigenetic mechanisms related to NCX1 and NCX3-induced neuroprotection in brain ischemic preconditioning could aid in discovering new epigenetic drugs that, by altering DNA or chromatin structure of NCX1 and NCX3 gene promoters, might reduce the infarct volume after a harmful ischemic insult.
Keywords: NCX1, NCX3, Neuroprotection, Epigenetic
Highlights
In the context of brain ischemic stroke and preconditioning, the expression of NCX1 and NCX3 can be triggered by epigenetic and chromatin modifications, which are able to activate neurosurvival pathways. Identifying the epigenetic regulation of the NCX1 and NCX3 promoters could allow for the identification of molecular targets useful to counteract ischemic stroke.
Introduction
Stroke is one of the leading causes of morbidity and mortality worldwide. In 2021, 7.44 million deaths were attributed to ischemic stroke (Wu et al., 2019; Wafa et al., 2020; Pu et al., 2023). Ischemic stroke results from transient or permanent occlusion of cerebral vessels; as a consequence of limited or absent blood supply, the affected brain regions experience oxygen and nutrient deprivation and, in the absence of an immediate intervention, become necrotic.
Stroke treatment involves three main approaches: management of the acute condition, post-stroke rehabilitation, and prevention interventions. Concerning prevention, in the past 30 years, ischemic preconditioning (PC) has attracted wide attention and in-depth research (Vijayakumar et al., 2016; Yang et al., 2020; Zhao et al., 2023). PC refers to a sub-lethal and non-injurious ischemic insult that can activate complex endogenous mechanisms to provide tolerance against a subsequent, otherwise lethal, ischemic insult. PC has gained widespread interest in clinical settings and neurological research, since it has been proposed as a potent and intriguing neuroprotective mechanism that facilitates the brain’s ability to cope with high levels of metabolic stress, including hypoxia or ischemia (Zhang et al., 2020; Akki et al., 2022; Shu et al., 2022). PC can be divided into two phases: (i) an early phase, which develops within a few minutes from the exposure to the insult (but lasts only one or two hours), and (ii) a late phase, which occurs within six to twelve hours (but lasts three to four days) (Li et al., 2017). The two phases confer neuroprotection through completely different mechanisms. The early phase is characterized by the modulation of ion channel permeability, the release of adenosine, nitric oxide (NO), and bradykinin, and post-translational modification of pre-existing proteins. The late phase is caused by de novo synthesis of proteins involved in cellular energy metabolism, immune response, endothelial health, and homeostasis (Koch et al., 2014). The exact mechanisms through which preconditioning exerts its beneficial effect remain uncertain; initially, it was proposed and demonstrated that the protection induced by PC in the heart also occurred via the regulation of Na+/Ca2+ balance (Steenbergen et al., 1993).
Over the years, it has been shown that the sodium-calcium exchanger (NCX) plays a pivotal role both in physiological and pathophysiological conditions in the brain. Specifically, two NCX isoforms, NCX1 and NCX3, are effectors of PC-induced neuroprotection. Indeed, both are targets for the survival action of the phosphatidylinositol 3-kinase (PI3-K)/Akt pathway (Brancaccio et al., 2022). Notably, the relevant role of NCX1 and NCX3 during preconditioning was further confirmed in vivo, when NCX1 and NCX3 knock-down induced by intracerebroventricular injections of siRNA in rats subjected to PC followed by transient middle cerebral artery occlusion (tMCAO), partially reversed preconditioning-induced neuroprotection (Pignataro et al., 2012).
Pharmacological or genetic strategies to enhance NCX1 and NCX3 expression and activity might represent a reasonable way to reduce neuronal damage after stroke. In this context, understanding the transcriptional and epigenetic regulations of NCX1 and NCX3 expression as effectors of neuroprotection evoked by PC is particularly intriguing.
Epigenetic control of NCX1 expression in cerebral ischemia and ischemic preconditioning
The solute carrier family (SLC) 8A1 gene encodes the NCX1 protein, which exchanges three Na+ for one Ca2+ across the cellular plasma membrane. NCX1 is ubiquitously expressed in mammalian cells, albeit NCX1 splicing variants are selectively expressed in a tissue-specific manner (Kofuji et al., 1994); in particular, NCX1 heart (Ht), kidney (Kd), and brain (Br) promoters are characterized by distinct binding sequences for several transcription factors, allowing for the tissue-restricted regulation of NCX1 expression (Quednau et al., 1997).
An epigenetic modification regulating Ht expression in the ischemic brain is DNA methylation. We recently found that the transcriptional factor RE1-silencing transcription factor (REST), by binding a REST consensus sequence named RE-1 element on Ht, generates a complex with the epigenetic reader methyl CpG binding proteins (MeCP2) and the epigenetic writer DNA-methyltransferase-1 (DNMT1), leading to the methylation of the Ht sequence. The effect of the REST/DNMT1/MeCP2 complex assembly is the downregulation of NCX1 expression in the peri-ischemic temporoparietal cortex (Guida et al., 2024).
In 2015, we reported another epigenetic regulation mechanism of NCX1 by two functional protein complexes: REST/Sp3/histone deacetylases (HDAC)1/HDAC2 and HIF-1/Sp1/p300 (Formisano et al., 2015). In particular, following a stroke, the transcriptional repressors Sp3 and REST interact with HDAC1 and HDAC2 on the Br, leading to the hypoacetylation of the promoter and the repression of the neuroprotective NCX1 gene. On the contrary, in brain preconditioning, we found that transcriptional activators Sp1 and HIF-1 co-localized with histone acetyltransferase (HAT) p300 on Br, leading to hyperacetylation of the promoter and NCX1 gene activation. Additionally, in primary cortical neurons subjected to an experimental protocol mimicking hypoxia, consisting of three hours of oxygen and glucose deprivation (OGD) plus 24 hours of reoxygenation (Rx), the effect of the class I HDAC inhibitor MS-275 is counteracted by the transfection of a siRNA against NCX1. In contrast, in neurons overexpressing NCX1 and subjected to the in vitro protocol of PC (PC+OGD/Rx), the neurotoxic effect of p300 inhibitor C646 is prevented (Figure 1A, Figure 2).
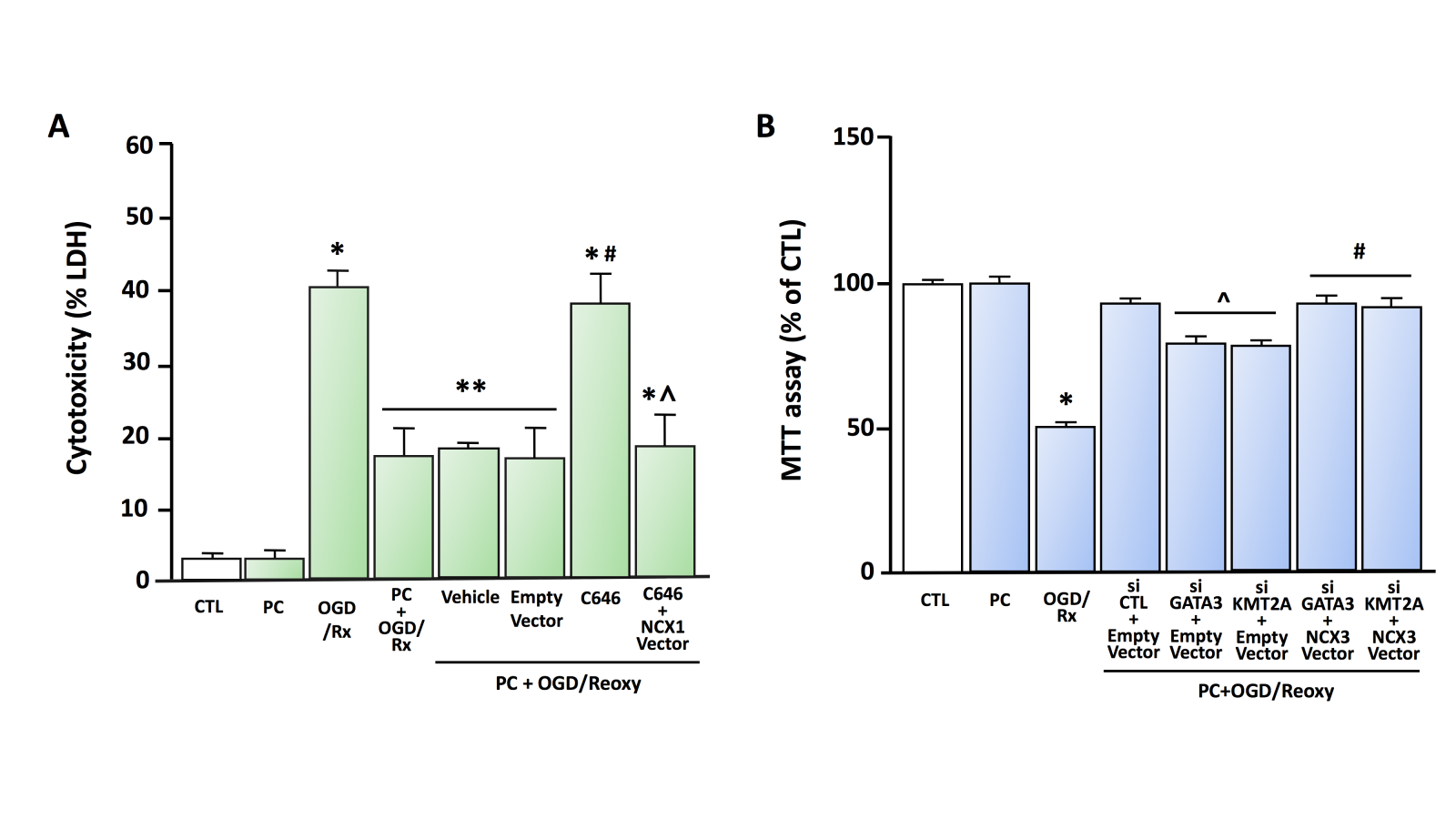
In a new window | Download PPT
Figure 1. A) Effect of HAT p300 inhibitor C646 on cell survival in cortical neurons exposed to OGD/Rx or PC+OGD/Rx (modified from Formisano et al., 2015) LDH assay was performed under the following experimental conditions: (1) CTL, (2) PC, (3) OGD/Rx, (4) PC+OGD/Rx, (5) PC+OGD/Rx+Vehicle, (6) PC+OGD/Rx+Empty Vector, (7) PC+OGD/Rx+C646, and (8) PC+OGD/Rx+C646+NCX1 vector. Each column represents the mean ± SEM. *p < 0.05 versus CTL. **p < 0.05 versus CTL, PC, OGD/Rx and PC+OGD/Rx+C646 #p < 0.05 versus PC+OGD/RX alone or with Vehicle and Empty Vector. ^p < 0.05 versus PC+OGD/RX+C646. Statistically significant differences between more than two experimental groups were evaluated by one-way ANOVA analysis followed by Bonferroni post-hoc test (n = 3). B) Effect of siRNA against GATA3 and KMT2A on cell survival in cortical neurons exposed to PC+OGD/Rx. MTT assay was performed under the following experimental conditions (from Guida et al., 2021 modified): (1) CTL, (2) PC, (3) OGD/Rx, (4) PC+OGD/Rx+siCTL+Empty Vector, (5) PC+OGD/Rx+siGATA3+Empty Vector, (6) PC+OGD/Rx+siKMT2A+Empty Vector, (7) PC+OGD/Rx+siGATA3+NCX3 Vector, (8) PC+OGD/Rx+siKMT2A+NCX3 Vector. Each column represents the mean ± SEM. *p<0.05 versus CTL, ^p<0.05 versus PC+OGD/Rx+siCTL+Empty Vector, #p<0.05 versus PC+OGD/Rx+siGATA3+Empty Vector and PC+OGD/Reoxy+siKMT2A+Empty Vector by one-way ANOVA analysis followed by Bonferroni post-hoc test (n = 4).
Epigenetic control of NCX3 expression in cerebral ischemia and ischemic preconditioning
The NCX3 gene consists of nine exons and possesses around 80% homology with the other NCX family isoforms, which also comprise NCX1 and NCX2 (Quednau et al., 1997). Since NCX3 knockdown increases the infarct size caused by stroke and inhibits the neuroprotection of the ischemic brain caused by preconditioning and postconditioning (Pignataro et al., 2012), NCX3 is considered a key player in the pathophysiology of stroke. Regarding the epigenetic mechanisms controlling the brain-restricted expression of NCX3 in the pathophysiology of stroke, we observed that HDAC4 and HDAC5, by physically interacting with the transcription factor downstream regulatory element antagonist modulator (DREAM), diminished NCX3 expression in primary cortical neurons. In addition, MC1568, a class IIA HDAC inhibitor selective for HDAC4 and HDAC5, considerably increased NCX3 expression by increasing the acetylation status of histone H4 on its promoter sequence (Formisano et al., 2020). More recently, unraveling the epigenetic mechanisms controlling NCX3 regulation in the PC, we found that the transcriptional activator GATA3, by binding the epigenetic writer KMT2A to the brain NCX3 promoter sequence, creates a complex that upregulates NCX3 expression. In particular, after brain PC, GATA3, by recruiting KMT2A, activates NCX3 gene transcription in the peri-infarct temporoparietal cortex, thereby reducing brain damage (Guida et al., 2021) (Figure 1B, Figure 2). With regard to other epigenetic mechanisms controlling NCX3 regulation, Cuomo et al. (2016) suggested that SUMO1 plays a pivotal role in neuroprotection elicited by PC; in particular, they show that SUMOylation levels increase during PC, thus preventing NCX3 degradation by the proteasome and mediating neuroprotection.
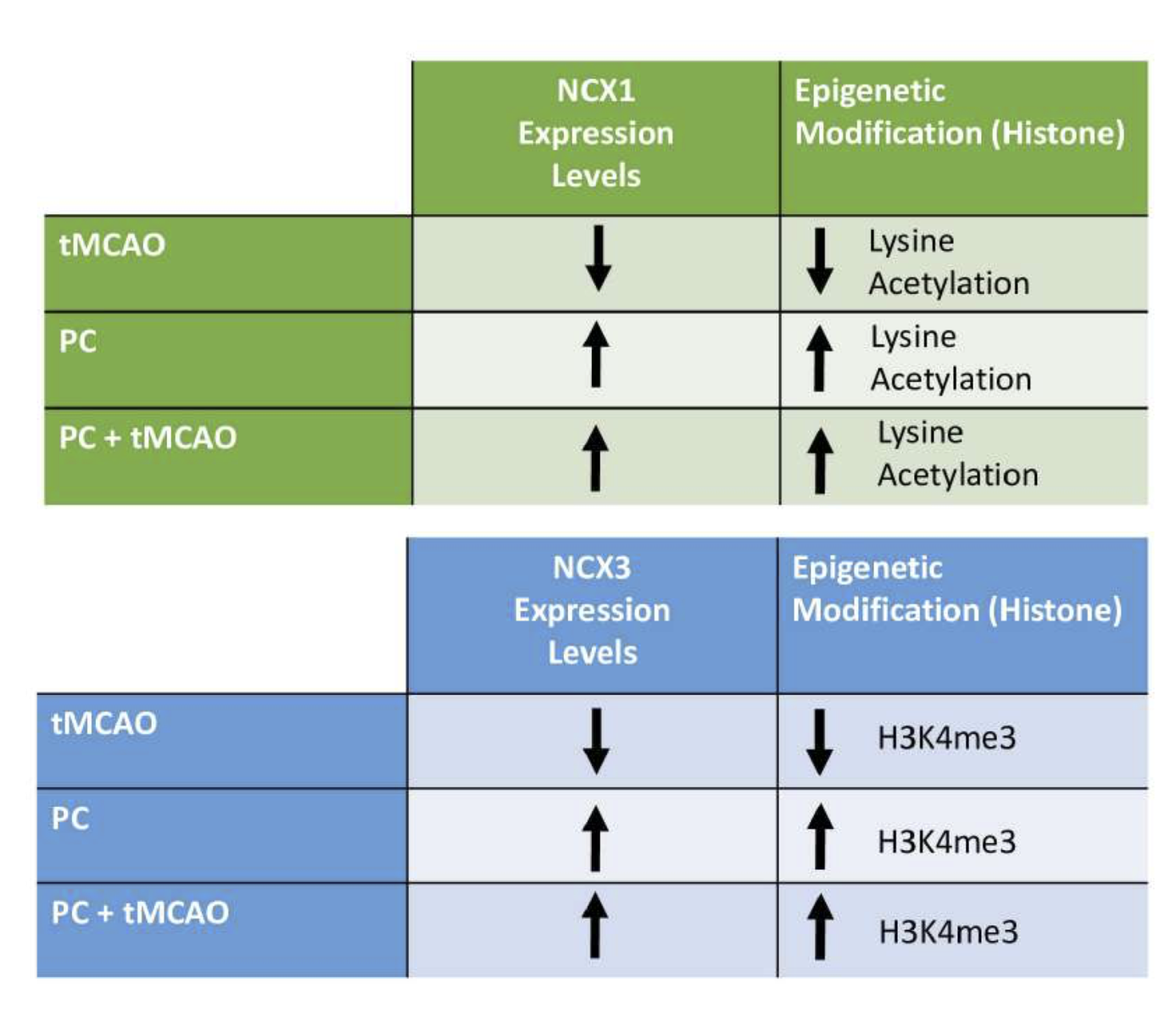
In a new window | Download PPT
Figure 2. Epigenetic modification and variation of NCX1 and NCX3 expression after ransient middle cerebral artery occlusion, ischemic preconditioning (PC) and PC plus tMCAO.
Conclusions
Epigenetic modifications and transcriptional regulation of sodium-calcium exchanger genes, particularly NCX1 and NCX3 isoforms, are fundamental to activating neurosurvival pathways. Interestingly, they are involved in a number of neurological conditions, such as Alzheimer’s disease (Preziuso et al., 2023), Parkinson’s disease (Sirabella et al., 2018), and amyotrophic lateral sclerosis (Petrozziello et al., 2022). In the context of PC, NCX1 and NCX3 play a pivotal role in mediating neuroprotection elicited by preconditioning. An effective stroke therapy could be designed by increasing the activity or inducing overexpression of both NCX1 and NCX3. In particular, drugs able to promote Sp1/HIF-1/histone acetyltransferase p300 and GATA3/KMT2A up-regulation to increase, respectively, NCX1 and NCX3 expression, might represent a new and innovative pharmacological strategy to reduce the infarct volume after a harmful ischemic insult.
Conflict of interests
The author(s) declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Sources of Funding
This work was supported by the following grant: MNESYS 2023 (PE0000006) by MIUR and National Recovery and Resilience Plan (NRRP) to Luigi Formisano.
References
Silvia Ruggiero
Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 8013, Naples, Italy.
Natascia Guida
Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 8013, Naples, Italy.
Luca Sanguigno
Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 8013, Naples, Italy.
Luigi Formisano
Division of Pharmacology, Department of Neuroscience, Reproductive and Dentistry Sciences, School of Medicine, Federico II University of Naples, Via Pansini, 5, 8013, Naples, Italy.
Corresponding author:
Silvia Ruggiero
Email: silviaruggiero98@gmail.com

In a new window | Download PPT
Figure 1. A) Effect of HAT p300 inhibitor C646 on cell survival in cortical neurons exposed to OGD/Rx or PC+OGD/Rx (modified from Formisano et al., 2015) LDH assay was performed under the following experimental conditions: (1) CTL, (2) PC, (3) OGD/Rx, (4) PC+OGD/Rx, (5) PC+OGD/Rx+Vehicle, (6) PC+OGD/Rx+Empty Vector, (7) PC+OGD/Rx+C646, and (8) PC+OGD/Rx+C646+NCX1 vector. Each column represents the mean ± SEM. *p < 0.05 versus CTL. **p < 0.05 versus CTL, PC, OGD/Rx and PC+OGD/Rx+C646 #p < 0.05 versus PC+OGD/RX alone or with Vehicle and Empty Vector. ^p < 0.05 versus PC+OGD/RX+C646. Statistically significant differences between more than two experimental groups were evaluated by one-way ANOVA analysis followed by Bonferroni post-hoc test (n = 3). B) Effect of siRNA against GATA3 and KMT2A on cell survival in cortical neurons exposed to PC+OGD/Rx. MTT assay was performed under the following experimental conditions (from Guida et al., 2021 modified): (1) CTL, (2) PC, (3) OGD/Rx, (4) PC+OGD/Rx+siCTL+Empty Vector, (5) PC+OGD/Rx+siGATA3+Empty Vector, (6) PC+OGD/Rx+siKMT2A+Empty Vector, (7) PC+OGD/Rx+siGATA3+NCX3 Vector, (8) PC+OGD/Rx+siKMT2A+NCX3 Vector. Each column represents the mean ± SEM. *p<0.05 versus CTL, ^p<0.05 versus PC+OGD/Rx+siCTL+Empty Vector, #p<0.05 versus PC+OGD/Rx+siGATA3+Empty Vector and PC+OGD/Reoxy+siKMT2A+Empty Vector by one-way ANOVA analysis followed by Bonferroni post-hoc test (n = 4).

In a new window | Download PPT
Figure 2. Epigenetic modification and variation of NCX1 and NCX3 expression after ransient middle cerebral artery occlusion, ischemic preconditioning (PC) and PC plus tMCAO.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 3523 | 7 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA