International bi-monthly journal of cell signaling, tissue protection, and translational research.
The potential of chronic remote ischemic conditioning in cerebral small vessel disease
David C. Hess1, Askiel Bruno1, Mohammad B. Khan1, Pradip Kamat1, Rolf Ankerlund Blauenfeldt2, Janne Kaergard Mortensen2, Grethe Andersen2, Wenbo Zhao3, Xunming Ji3
Author Affiliations


- 1Department of Neurology, Medical College of Georgia, Augusta University.
- 2Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
- 3Department of Neurology, Xuanwu Hospital, Capitol Medical University, Beijing, China.
Abstract
Chronic remote ischemic conditioning (C-RIC) is the month-long or more intervention of RIC. C-RIC has shown promise in a large randomized clinical trial in intracranial atherosclerotic stenosis (ICAS) to reduce the risk of stroke. C-RIC has the potential to reduce cognitive impairment in cerebral small vessel disease (cSVD). C-RIC is effective in a preclinical model of vascular cognitive impairment and dementia (VCID), the bilateral carotid stenosis model in mice, at reducing cognitive impairment, and white matter damage. C-RIC is safe and shows promise in pilot clinical trials of cSVD. In cohort studies, physical exercise is effective at preventing stroke in ICAS and in slowing the progression of cognitive decline in cSVD. C-RIC shares common mechanisms with physical exercise and is an attractive therapy for cSVD. Dosing and compliance are two issues that need to be resolved before large phase II-III trials in cSVD can be optimized.
Keywords: Remote ischemic conditioning, Cerebral small vessel disease, Intracranial atherosclerotic stenosis, Biomarkers, Clinical trial
Abstract
Chronic remote ischemic conditioning (C-RIC) is the month-long or more intervention of RIC. C-RIC has shown promise in a large randomized clinical trial in intracranial atherosclerotic stenosis (ICAS) to reduce the risk of stroke. C-RIC has the potential to reduce cognitive impairment in cerebral small vessel disease (cSVD). C-RIC is effective in a preclinical model of vascular cognitive impairment and dementia (VCID), the bilateral carotid stenosis model in mice, at reducing cognitive impairment, and white matter damage. C-RIC is safe and shows promise in pilot clinical trials of cSVD. In cohort studies, physical exercise is effective at preventing stroke in ICAS and in slowing the progression of cognitive decline in cSVD. C-RIC shares common mechanisms with physical exercise and is an attractive therapy for cSVD. Dosing and compliance are two issues that need to be resolved before large phase II-III trials in cSVD can be optimized.
Keywords: Remote ischemic conditioning, Cerebral small vessel disease, Intracranial atherosclerotic stenosis, Biomarkers, Clinical trial
Highlights
C-RIC was safe and showed benefit at preventing stroke in compliant patients in a large randomized clinical trial in ICAS. In observational studies, physical exercise reduces recurrent stroke in patients with ICAS and slows cognitive decline in cSVD. Physical exercise shares common mechanisms with C-RIC. C-RIC is a promising therapy in cSVD as a preclinical model in mice showed a reduction in cognitive impairment and white matter damage and small pilot clinical trials in cSVD show safety and hints of activity. Large phase II-III clinical trials of C-RIC in cSVD are needed after dosing and compliance are optimized.
Introduction
Remote ischemic conditioning (RIC) is the repeated brief episodes of non-lethal ischemia of a distant organ, such as the limb, to protect the heart and brain against lethal ischemia. RIC is a promising treatment for acute stroke and for the prevention of stroke in patients with intracranial atherosclerotic stenosis (ICAS) (Chen et al., 2022; Hou et al., 2022). However, clinical trials have shown mixed results when RIC was performed only in the acute (days after the event) phase (Blauenfeldt et al., 2023) or subacute (weeks) phase of stroke (Chen et al., 2022). Chronic RIC (C-RIC) uses long-term repetitive RIC to prevent vascular events and the progression of the disease. RIC shares common mechanisms with physical exercise (Hess et al., 2015; Zhao et al., 2018). In an analogy to physical exercise, acute RIC is similar to short-term exercise-induced protection against ischemic-reperfusion such as myocardial infarction and stroke, while C-RIC is similar to longer-term habitual exercise protection to prevent cardiovascular events (Thijssen et al., 2022).
C-RIC in intracranial atherosclerotic stenosis (ICAS)
ICAS is one of the most common causes of stroke in the world and has a high rate of stroke recurrence compared to other stroke subtypes. It is more prevalent in Asians than Whites (Hoh and Chimowitz, 2024). ICAS is associated with a high risk of cognitive impairment and dementia (Sabayan et al., 2023). The Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) clinical trial randomized patients to aggressive medical management (AMM; i.e., combination antiplatelet therapy, intensive risk factor management, and lifestyle modification) or to intracranial stenting plus AMM. The 30-day rate of stroke in the stenting arm of SAMMPRIS was exceedingly high (14.7%) and resulted in enrollment in the trial being stopped early (Chimowitz et al., 2011; Derdeyn et al., 2014). However, the one-year rate of recurrent symptomatic cerebral infarction in the territory of the stenotic artery in patients with a previous symptomatic cerebral infarct (74% of the SAMMPRIS cohort) was still very high (19.7%) despite AMM. One of the lessons learned from the SAMMPRIS trial was the benefit of physical exercise in ICAS. Physical activity was the strongest predictor of a good outcome in the AMM arm of the SAMMPRIS trial (Turan et al., 2017). The recommendations for optimal medical management in ICAS include combination antiplatelet drugs, blood pressure control, target low-density lipoprotein (LDL) <70 mg/dl, hemoglobin (Hgb) A1c less than 7%, and physical activity (Wabnitz and Turan, 2024).
The Chronic Remote Ischaemic Conditioning In Patients With Symptomatic Intracranial Atherosclerotic Stenosis (RICA) trial was a multicenter, double-blind, clinical trial of 3033 subjects with ICAS randomized to C-RIC (daily bilateral arm conditioning versus sham-control for 12 months) at 84 stroke centers in China (ClinicalTrials.gov, number NCT02534545) (Hou et al., 2022). The trial included patients aged 40-80 years with ischemic stroke or transient ischemic attack (TIA) attributable to angiographically verified 50–99% stenosis. The primary efficacy endpoint was the time to first occurrence of non-fatal or fatal ischemic stroke. Prespecified secondary efficacy endpoints included the composite of time to first occurrence of any stroke (ischemic or hemorrhagic), TIA, or myocardial infarction.
In the intent to treat population, C-RIC non-significantly reduced the primary outcome of ischemic stroke, with a hazard ratio 0·87 (95% CI 0·74-1·03; p = 0·12). C-RIC reduced the secondary composite outcome of total stroke, TIA, and myocardial infarction, with a hazard ratio of 0·82 (95% CI 0·71-0·95; p = 0·0089). In the prespecified per-protocol analysis, in patients performing the RIC intervention at least 50% of the potential treatment days in the first 12 months, C-RIC significantly reduced the occurrence of ischemic stroke. C-RIC also significantly reduced the secondary composite outcome of stroke (ischemic and hemorrhagic), TIA, and myocardial infarction. Compliance with RIC was an issue in this trial. Only 46.5% of the subjects were at least 50% compliant during the first 12 months.
The encouraging feature of the RICA trial was the “activity” of C-RIC in a disease that has no established treatment beyond AMM. One of the lessons learned from this trial is the long-term poor compliance of C-RIC. This compliance issue will need to be solved in future clinical trials and before RIC is used clinically. Some ways to improve compliance in future clinical trials are gamification and financial incentives to motivate patients to use the therapy (Agarwal et al., 2021). The authors of the RICA trial also have adapted the bilateral conditioning device to a vest to provide ambulatory RIC.
Cerebral small vessel disease
Dementia is one of the major threats to world public health, and there are fears of an incoming tsunami of vascular dementia (Greenberg, 2017). Vascular dementia is estimated to be responsible for about 30% of total dementia cases and contributes to another 20%. Some form of vascular pathology is seen in 80% of cases of sporadic Alzheimer’s disease (AD) (Toledo et al., 2013). In the Religious Orders Study and Rush Memory and Aging cohorts, cerebral atherosclerosis and arteriosclerosis increased the risk of AD (Arvanitakis et al., 2016). These vascular contributions to cognitive impairment and dementia are known by the acronym, VCID, and the most common subtype is cerebral small vessel disease (cSVD) (Markus et al., 2022). The pathological hallmarks of cSVD are lacunes and white matter damage (WMD) from ischemia in the periventricular regions and centrum semiovale. There are two forms of cSVD: 1) an arteriosclerotic form, often but not always accompanied by hypertension, and 2) cerebral amyloid angiopathy. WMD is mediated by blood-brain barrier (BBB) leakage, microglial activation, and injury to oligodendrocytes and myelin. WMD is best detected by magnetic resonance imaging (MRI) (Markus et al., 2022). The most sensitive MRI marker and best predictor of dementia is diffusion tensor imaging (DTI), which measures disruption of WM architecture and disconnection of brain networks (Egle et al., 2022).
Currently, there is no proven treatment to slow down the progression of cSVD. With the aging of the US and world population, the disability from cSVD is expected to grow. Other than control of vascular risk factors (e.g., hypertension), there is no known effective targeted treatment of cSVD. There have been relatively few randomized clinical trials in cSVD (Markus et al., 2022). The Leukoaraiosis and Disability Study (LADIS Study), a prospective multinational European cohort study that evaluated the impact of WMD on elderly people living independently, found that physical activity reduced the risk of cognitive decline and dependency (Verdelho et al., 2012).
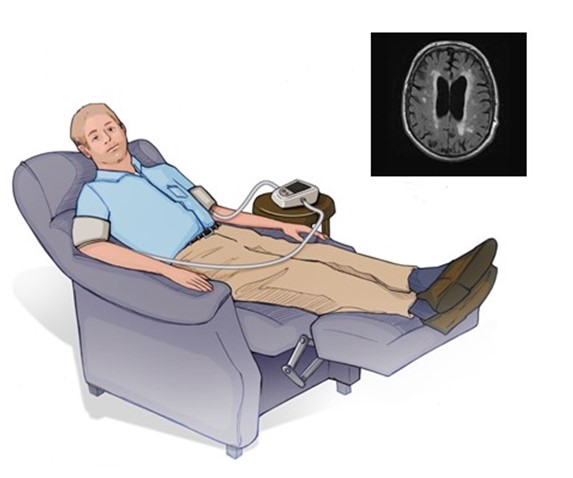
In both cSVD and ICAS observational cohort studies, physical exercise reduces critical clinical endpoints such as cognitive decline and recurrent stroke. In 2020, only a quarter of Americans met the 2018 guidelines for physical activity, and older individuals are less likely to exercise than younger individuals (Elgaddal et al., 2020). Since many patients are unwilling or unable to exercise, an alternative is to develop “exercise equivalents” such as C-RIC (Figure 1).
C-RIC is effective in preclinical models of VCID. The bilateral carotid artery stenosis (BCAS) model produces chronic hypoperfusion and is regarded as the most valid mouse model for VCID (Bink et al., 2013). Mice develop WMD and cognitive impairment. The BCAS model at six months shows features of human cSVD with increased vessel width with collagen IV and fibrin deposition in the vessel wall and luminal narrowing (Holland et al., 2015; Duncombe et al., 2017). Moreover, there is a disruption of aquaporin IV that plays a key role in the glymphatic system (Holland et al., 2015). C-RIC daily for two weeks increased cerebral blood flow (CBF), improved cognition and functional outcomes, and reduced WMD at one month compared to sham C-RIC (Khan et al., 2015). C-RIC administered daily for one or four months also improved long-term outcomes at six months (Khan et al., 2018). Both one month and four months of daily C-RIC improved CBF, induced increased angiogenesis and arteriogenesis, reduced WMD, and improved cognition at six months in young male mice. Daily forced treadmill exercise is also effective in the BCAS model at improving CBF, reducing WMD, and improving cognition (Khan et al., 2024).
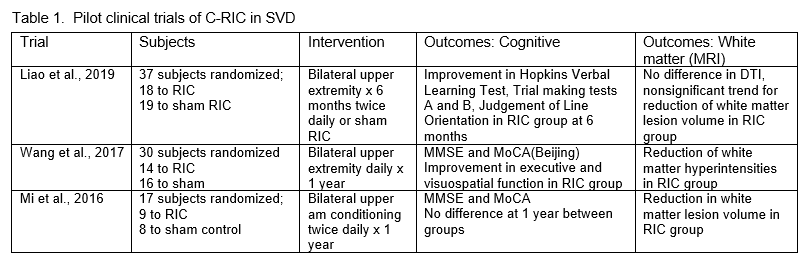
There is also some clinical trial evidence that C-RIC may be beneficial in SVD. Three small pilot clinical trials of C-RIC in cSVD were shown to be safe, and there was some suggestion of benefit (Table 1) (Mi et al., 2016; Wang et al., 2017; Liao et al., 2019). A post hoc stroke subgroup analysis from the Remote Ischemic Conditioning in Patients With Acture Stroke (RESIST) acute stroke trial showed that in patients with the small vessel disease subtype of stroke who maintained good treatment adherence, RIC showed functional benefit at 90 days (Blauenfeldt et al., 2024).
Two critical issues remain to optimize clinical trials of C-RIC in cSVD: compliance and dosing. Compliance with the intervention can be improved by daily text reminders and telemetric monitoring. Gamification, effective in some exercise trials, may also increase compliance (Agarwal et al., 2021). In a recent small randomized pilot study, increased age and reduced cognitive function at baseline were associated with reduced short-term treatment compliance (Kjolhede et al., 2024). This may represent a translational issue as subjects with older age and cognitive difficulty would be the target cSVD population.
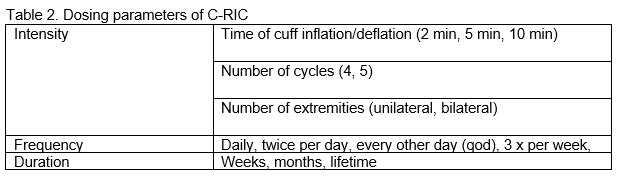
Dosing of RIC, like physical exercise, can be divided into intensity, frequency, and duration (Table 2). In a disease such as cSVD, treatment would likely have to last years. One possibility would be a reduction of dose frequency after a year - e.g., every other day or even twice per week. Most preclinical models in acute stroke and BCAS use bilateral lower extremity conditioning. The RICA trial, which was positive in the per-protocol analysis of compliant subjects, and the RICAMIS trial, which showed beneficial effects in acute stroke patients, used bilateral upper extremity conditioning (Chen et al., 2022; Hou et al., 2022). The RESIST trial used unilateral arm conditioning with neutral effects (Blauenfeldt et al., 2023).
There are no established plasma and blood biomarkers for response to C-RIC. In patients with ICAS, C-RIC increased CBF as measured by single-photon emission computerized tomography (Meng et al., 2012). In the BCAS preclinical models, C-RIC increases CBF and angiogenesis. MRI arterial spin labeling may have the potential to image changes in CBF in response to RIC.
The Chronic Remote Ischemic Conditioning in Vascular Cognitive Impairment: A Dose Escalation Study (ClinicalTrials.gov NCT06179797) is an ongoing early-phase clinical trial that involves 40 subjects with cerebrovascular symptoms, TIA, or mild ischemic stroke. Subjects are older than 55 years, free from dementia, and had a brain MRI within six months of enrollment with Fazekas score (a measure of white matter lesion burden) of 0-2. RIC involves bilateral upper arm cuff inflations to 50 mmHg above the systolic blood pressure with four on-off cycles lasting 2.5 to 5.0 minutes for one month. Blood biomarkers are measured, including red blood cell deformability, plasma nitrite, and monocyte polarization.
Five dose escalating treatment tiers are tested with eight subjects per group:
Tier 1: Sham treatment consisting of daily sub-systolic cuff inflation to 50 mmHg, 2.5-minute on-off cycles.
Tier 2: Cuff inflations every other day, 2.5-minute on-off cycles.
Tier 3: Cuff inflations every other day, 5.0-minute on-off cycles.
Tier 4: Cuff inflations once daily, 5.0-minute on-off cycles.
Tier 5: Cuff inflations twice daily, 5.0-minute on-off cycles.
The two co-primary outcomes are changes in blood inflammatory biomarkers (M1:M2 monocyte polarization) and red blood cell deformability after four weeks of treatment followed by one week off treatment.
We encourage more dose escalation and dose finding studies of C-RIC in cSVD. While coordinating with funding agencies is always challenging, an international collaboration enrolling North American, European, and Asian patients will provide a heterogeneous population. Once the dose and compliance are optimized in early phase studies, phase II-III adaptive design trials with surrogate imaging in phase II and measurements of cognitive decline in phase III are needed. Using surrogate markers such as MRI will allow sample sizes of about 130-150 per group with an effect size of 30% over three years (Benjamin et al., 2016; Smith and Markus, 2020). These can be developed as go/no go criteria for a phase III trial with cognitive decline as the primary outcome.
In a new window | Download PPT
Figure 1. Depiction of bilateral arm RIC in the home setting using a remote conditioning device.
Acknowledgment
We acknowledge Colby Zahn, MS MI (Master’s Medical Illustration) for Figure 1. David C. Hess received funding from the National Institute of Health (NIH 4R01NS12286).
Conflict of interest
Rolf Blauenfeldt reported receiving lecture fees from Bayer, Pfizer, and Novo Nordisk outside the submitted work. David C. Hess has a patent with Athrsys/Healios with royalties through Augusta University outside the submitted work. Askiel Bruno, Mohammad Khan, Pradip Kamat, Janne Kaergard Mortensen, Grethe Andersen, Wenbo Zhao, and Xunming Ji report no conflict of interest.
References
David C. Hess1
1Department of Neurology, Medical College of Georgia, Augusta University.
Askiel Bruno1
1Department of Neurology, Medical College of Georgia, Augusta University.
Mohammad B. Khan1
1Department of Neurology, Medical College of Georgia, Augusta University.
Pradip Kamat1
1Department of Neurology, Medical College of Georgia, Augusta University.
Rolf Ankerlund Blauenfeldt2
2Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Janne Kaergard Mortensen2
2Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Grethe Andersen2
2Department of Neurology, Aarhus University Hospital and Clinical Medicine, Aarhus University, Aarhus, Denmark.
Wenbo Zhao3
3Department of Neurology, Xuanwu Hospital, Capitol Medical University, Beijing, China.
Xunming Ji3
3Department of Neurology, Xuanwu Hospital, Capitol Medical University, Beijing, China.
Corresponding author:
David C. Hess
Email: dhess@augusta.edu

In a new window | Download PPT
Figure 1. Depiction of bilateral arm RIC in the home setting using a remote conditioning device.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 3612 | 16 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA