International bi-monthly journal of cell signaling, tissue protection, and translational research.
Effects of aging and comorbidities on endothelial progenitor cells
Jesús M. Pradillo1, Alba Grayston2, Violeta Medina-Alonso1, Mercedes Arrúe2, Ignacio Lizasoain1, Anna Rosell2
Author Affiliations


- 1Departamento de Farmacología y Toxicología, Facultad de Medicina, Universidad Complutense de Madrid (UCM), Instituto de Investigación Hospital 12 de Octubre (i+12), Madrid, Spain.
- 2Neurovascular Research Laboratory and Neurology Department, Vall d’Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Abstract
Endothelial Progenitor Cells (EPCs) are postulated to participate in the function of the vessel endothelium and are emerging as key cellular elements of vessel and tissue repair. These bone marrow-derived cells play a central role in maintaining blood vessel health and function under physiological conditions. Several investigations have identified these cells as mediators of repair; however, their number and function vary among subjects according to age, sex, and comorbid circumstances, which may negatively condition the characteristics and functions of EPCs. Lifestyle habits in developed countries increase the prevalence of comorbid conditions, including cardiovascular diseases and disorders in which the health of the vascular system is compromised. In this review, we describe how age, diabetes, hypertension, and obesity (significant comorbid conditions) may influence the opportunities for vascular remodeling by modifying the bio-distribution and functions of EPCs. We provide evidence for the need to improve cardiovascular protection beyond endothelial function and the vascular wall structure by preserving the endogenous mechanisms of vascular repair based on the actions of the endothelial progenitor pools.
Keywords: endothelial progenitor cells, stroke, cardiovascular disease, aging, comorbidity, vascular repair
Abstract
Endothelial Progenitor Cells (EPCs) are postulated to participate in the function of the vessel endothelium and are emerging as key cellular elements of vessel and tissue repair. These bone marrow-derived cells play a central role in maintaining blood vessel health and function under physiological conditions. Several investigations have identified these cells as mediators of repair; however, their number and function vary among subjects according to age, sex, and comorbid circumstances, which may negatively condition the characteristics and functions of EPCs. Lifestyle habits in developed countries increase the prevalence of comorbid conditions, including cardiovascular diseases and disorders in which the health of the vascular system is compromised. In this review, we describe how age, diabetes, hypertension, and obesity (significant comorbid conditions) may influence the opportunities for vascular remodeling by modifying the bio-distribution and functions of EPCs. We provide evidence for the need to improve cardiovascular protection beyond endothelial function and the vascular wall structure by preserving the endogenous mechanisms of vascular repair based on the actions of the endothelial progenitor pools.
Keywords: endothelial progenitor cells, stroke, cardiovascular disease, aging, comorbidity, vascular repair
1. Introduction
Cardiovascular diseases (CVDs) comprise a group of disorders of the heart and blood vessels, including coronary heart disease, cerebrovascular disease, and peripheral arterial disease, among others. These diseases cause 17.9 million deaths every year and account for 31% of all global deaths, according to the World Health Organization (WHO). CVDs primarily manifest as heart attacks and strokes, which are associated with several lifestyle habits, including tobacco use, an unhealthy diet, physical inactivity, and alcohol abuse. Such lifestyle choices increase the prevalence of raised blood pressure, elevated blood glucose, high cholesterol, and obesity, which are risk factors that negatively affect cardiovascular health primarily by damaging and narrowing or obstructing the blood vessels (Beaglehole, 2001). Apart from these direct effects on the vascular walls, the presence of one or multiple comorbid conditions can influence other organs, cellular metabolism and function, tissue repair mechanisms, or the number and function of stem/progenitor cells. Therefore, the preservation of endothelial function and blood vessel health is essential for the prevention of the progression of CVDs, both in aged and young individuals.
A dysfunctional or damaged endothelium, with limited regenerative capacity, leads to platelet aggregation, unbalanced hemostasis, immune cell infiltration, increased oxidative stress, reduced vasomotor capabilities, and cellular turnover of the blood vessels (Ross, 2018; Widmer and Leman, 2014). A dysfunctional or damaged endothelium is also associated with arterial atherosclerosis, blood clot formation, reduced vessel repair, and impaired angiogenesis or tissue remodeling. In this context, endothelial progenitor cells (EPCs), also known as circulating angiogenic cells, which derive from bone marrow (BM), play a central role in maintaining blood vessel health and function (Esquiva et al., 2018) in the following ways: EPCs provide a source for physiological endothelial cell replacement; EPCs provide a source for endothelial repair via incorporation into the damaged vasculature as mature endothelial cells; EPCs promote angiogenesis by stimulating other endothelial cells to proliferate; and EPCs promote whole tissue remodeling in the recovery phases of stroke or cardiac injury. However, the number and function of EPCs may be negatively conditioned by the presence of the abovementioned risk factors. In this review, we highlight the importance of EPCs as key cellular elements of endothelial function and vessel and tissue repair. The influence of aging and comorbid conditions, which are associated with CVD, on EPC function, will also be discussed.
2. Endothelial Progenitor Cells
2.1. Background: EPC populations and functions
The formation of new blood vessels was once thought to result from embryonic vasculogenesis followed by an outbreak of endothelial cells from existing vessels (Pepper, 1997). However, this dogma was questioned with the discovery of BM-derived EPCs in adult peripheral blood, which were first identified as CD34 antigen-positive (CD34+) mononuclear cells (MNC) with endothelial characteristics (Asahara et al., 1997). The proportion of these cells generally ranges between 0.1 and 2% of the total MNCs in the BM, peripheral blood, and cord blood. EPCs are mobilized from the BM into the peripheral blood as an endogenous response to the physiopathological demands of neovascularization and can be differentiated into functional endothelial cells in vitro and ex vivo. Currently, these cells play an important role in adult vasculogenesis and angiogenesis by participating not only in the formation of vessels but also in vessel repair and remodeling (Liman and Endres, 2012). However, the identification of EPCs remains controversial, as no single marker has been identified for this type of cell. Currently, these cells are defined as cells expressing both stem cell and endothelial markers, and flow cytometry is the most commonly applied approach to count EPCs. Most accepted definitions describe EPCs as proliferating cells that express the cell-surface markers of stem cells (CD34 or CD133) and endothelial cells (vascular endothelial growth factor (VEGF) receptor-2) (Liman and Endres, 2012), although other progenitor or endothelial markers have also been used ((Fadini et al., 2012). Despite the lack of a simple and rapid marker for identifying EPCs, there is consensus regarding the classification of these cells into two major types that emerge from MNC cultures, which were initially named “early EPCs” or circulating angiogenic cells (CAC) and “late EPCs” or outgrowth endothelial cells (OEC). These types of cells have distinct origins and contribute differently to angiogenesis (Fadini et al., 2012; Medina et al., 2017). The early EPCs appear rapidly in culture under specific conditions for endothelial cell growth, and the late EPCs appear after 2 to 3 weeks and expand in culture. While the early-EPCs exhibit a spindle shape within heterogeneous populations of cells, the late-OECs or endothelial colony-forming cells (ECFCs) exhibit cobblestone colonies with a characteristic clonogenic capacity (Hur et al., 2004; Gulati et al., 2003). Regardless of these controversies, it has been demonstrated that EPCs are capable of differentiating ex vivo into endothelial-like cells and therefore represent a new model for endothelial regeneration, vessel repair, and angio-vasculogenesis.
In addition to the therapeutic interest in endothelial progenitors for vascular regeneration due to their homing and engraftment into new or damaged blood vessels, it is also known that EPCs secrete a large number of growth factors, which contribute to vessel and tissue regeneration through stromal cell-derived factor 1 (SDF-1), VEGF, granulocyte-colony stimulating factor,, endothelial nitric oxide synthase (eNOS), inducible NOS (iNOS), interleukin (IL)-8, and several matrix metalloproteinases (MMPs), among many others (Ma et al., 2015). These factors can promote endothelial cell proliferation and migration, reduce cell apoptosis, regulate the recruitment of endogenous progenitor cells, and promote vascular growth and remodeling (Urbich et al., 2005; Di Santo et al., 2009; Rosell et al., 2013). Moreover, the release of these factors appears to be increased by hypoxia; for example, Di Santo and colleagues showed that angiogenin, hepatocyte growth factor, IL-8, platelet-derived growth factor-BB, SDF-1, and VEGF-A are increased in EPC-conditioned media under hypoxic conditions (Di Santo et al., 2009), which supports the protective role of EPCs in the context of cerebral ischemia.
2.2. EPCs in tissue repair and their role in stroke neurorepair
Stroke is a CVD with a clear need for new therapies beyond the acute phase of the disease, during which reperfusion therapies have already demonstrated a clear impact on improving stroke management and neuroprotection (Hacke et al., 2005; Urra et al., 2015; Nogueira et al., 2017). The nature of this devastating disease leads to severely damaged tissue/cells in very close proximity to healthy peri-infarct tissue. The classical view of neuronal rescue/repair has changed in the last decades into a more global understanding of the brain as a whole. This view has expanded to include other cell types (glial cells, inflammatory cells, stem/progenitor cells), the extracellular matrix, and the communication between these components. Therefore, the endogenous mechanisms of neurovascular repair include angio-vasculogenesis (formation of new blood vessels), gliogenesis (formation of new glial cells), neurogenesis (formation of new neurons), remyelination (new myelin sheaths on demyelinated axons), among others. Several of these endogenous mechanisms are activated in the minutes following the ischemic trigger in peri-infarct areas (Lo, 2005; Carmichael, 2008). Several populations of newborn progenitor cells have been identified in remodeling areas (Ohab et al., 2006; Jin et al., 2006), such as neural progenitor cells (NPCs), EPCs, or oligodendrocyte progenitor cells (OPCs). These findings demonstrate the plastic nature of the brain, in contrast to the more classical views of brains passively dying.
The following two elements must be highlighted in the natural neurorepair process: the neurovascular coupling and the neurovascular niche (Shen et al., 2004; Ohab et al., 2006). The former refers to the neurogenesis phenomenon which is closely linked to post-stroke angiogenesis, and the latter refers to the factors released by the tissue cells, which create an appropriate niche for neurorepair. The importance of this coupling was demonstrated when ischemic mice that received systemic endostatin to inhibit angiogenesis abolished the normal patterns of neuroblast migration through the peri-infarct areas from the subventricular zone (Ohab et al., 2006). The authors of that study elegantly demonstrated that the vascular production of SDF-1 and angiopoietin 1 (Ang1) promote neuroblast migration to peri-infarct cortex (newly generated neurons expressing C-X-C chemokine receptor type (CXCR4) or Tie-2, which are the receptors for SDF-1 and Ang1, respectively). Meanwhile, other authors have identified proliferating neuroblasts in peri-infarct areas of the human brain in close proximity to blood vessels (Jin et al., 2006). Moreover, it has been described that the trophic support of endothelial cells to the neural stem cell niche also occurs in non-pathological conditions, wherein brain endothelial cells secrete soluble factors that maintain CNS stem cell self-renewal and neurogenic potential in vitro (Shen et al., 2004). The crosstalk between the endothelium and oligodendrocyte/oligodendrocyte progenitors, which involves nourishing trophic factors that are released by endothelial cells, has also been described in the normal and ischemic brain, wherein brain derived neurotrophic factor, transforming growth factor-beta, VEGF or MMPs may be responsible for maintaining the oligovascular niche (Miyamoto et al., 2014; Pham et al., 2012).
In this context, increasing evidence demonstrates that EPCs are present after ischemic stroke. The first studies of EPC mobilization in response to tissue ischemia (Takahashi et al., 1999) showed that this process was potentially related to the maintenance of endothelial integrity and the need for vascular remodeling. Other studies have demonstrated the incorporation of EPCs into neovessels (Asahara et al., 1997), and Taguchi and colleagues first reported that CD34+/CD133+ cells, as an EPC-enriched population, were a marker of cerebrovascular function (Taguchi et al., 2004). Further research in the field demonstrate that, following injury, unidentified signals induce EPC mobilization in the blood and produce a variety of growth factors as well as cytokines, which recruit EPCs to the injury site for neovascularization and tissue repair (Reinisch et al., 2009; Navarro-Sobrino et al., 2010; Massot et al., 2013). One of the most relevant signaling systems that participates in the endogenous recruitment of EPCs to the injured tissue is the CXCR4/SDF-1 axis, which is activated in animal models of middle cerebral artery occlusion (MCAO) in rodents (Mao et al., 2014). In humans, studies show that after stroke, there is an increase in circulating EPCs in the acute phases, which is related to better outcomes (Navarro-Sobrino et al., 2010; Pías-Peleteiro et al., 2016), and is followed by a decrease in the EPC counts at 3 months (Martí-Fábregas et al., 2013). Moreover, a recent study suggests that the initial stroke-driven increase in EPCs could be maintained in response to post-stroke rehabilitation therapy during the months after the ischemic insult (Gabriel-Salazar et al., 2018).
2.3. Cell-therapy opportunities involving EPCs
Transplantation of EPCs has emerged as a promising approach to enhance tissue repair. Initial studies demonstrate that EPC therapy enhance angio-vasculogenic responses in ischemic conditions. However, later studies demonstrate that these cells are capable of communicating with other cell types to participate in other repair mechanisms beyond vessel remodeling, including neurogenesis, in the context of stroke (Ma et al., 2015). EPCs can be mobilized in response to a hypoxic event, can home in to sites of neovascularization, and can differentiate into endothelial cells. They can also secrete growth factors and exosome bodies in cell-to-cell communication mechanisms (Urbich et al., 2005; Esquiva et al., 2018).
Although many pre-clinical studies support the benefits of EPC treatments for stroke by enhancing vascular remodeling and neurorepair in the context of stroke, there is still a lack of trials investigating the safety and/or efficacy of EPC therapy. However, even without trials several therapeutic approaches are possible, including autologous transplantation after blood extraction or BM aspiration, posterior cell isolation and/or expansion, heterologous cell administration of EPCs obtained from healthy donors, and even the administration of EPC-secreted factors in a cell-free strategy. However, clinical data that evaluate the best strategy are still missing. Moreover, with the new endovascular treatments to remove cerebral blood clots in stroke patients, new therapeutic approaches can now be considered to physically deliver the therapeutic product (e.g. EPCs or EPCs-secreted factors) directly to the affected brain territory. However to date, one Phase I clinical trial has confirmed the safety and feasibility of administering intra-arterially autologous BM-derived CD34+ cells (which includes the EPC population) in a small number of stroke patients (Banerjee et al., 2014). Most recently, a new randomized controlled Phase IIa trial has been initiated to investigate the reduction in infarct volume in stroke patients who receive intra-arterial CD34+ cells in the acute phase (Sargento-Freitas et al., 2018).
Finally, growing evidence suggest that the regenerative potential of the cell therapies that are being currently investigated are, in part, orchestrated by the proteins secreted by the cells (the secretome), which reflect the functional competence of a given cell. The secretome is also responsible for the crosstalk between cells and with the extracellular matrix via trophic factors, cytokines, or other bioactive molecules. In this regard, cell-free strategies have been proposed for regenerative medicine, and several authors have already proposed to treat cerebral ischemia (Rosell et al., 2013), wound healing in the context of diabetes (Kim et al., 2010), hindlimb ischemia (Di Santo et al., 2009) and, more recently, white matter damage induced by chronic hypoperfusion (Maki et al., 2018). The use of these cell-based but cell-free EPC strategies warrants further testing in the stroke clinical setting.
3. Comorbid conditions and EPCs
Aging is an inevitable process characterized by the progressive degeneration of tissue and organ systems, the deterioration of body functions, and the declining ability to respond to stress. Aging can also trigger the emergence of other risk factors and, consequently, increase the risk of age-related diseases. These comorbidities include hypertension, diabetes, obesity, atherosclerosis, CVD, and stroke, and their development accelerates and aggravates the process of aging, leading to disability and premature death (Tian and Li, 2014; Bao et al., 2014).
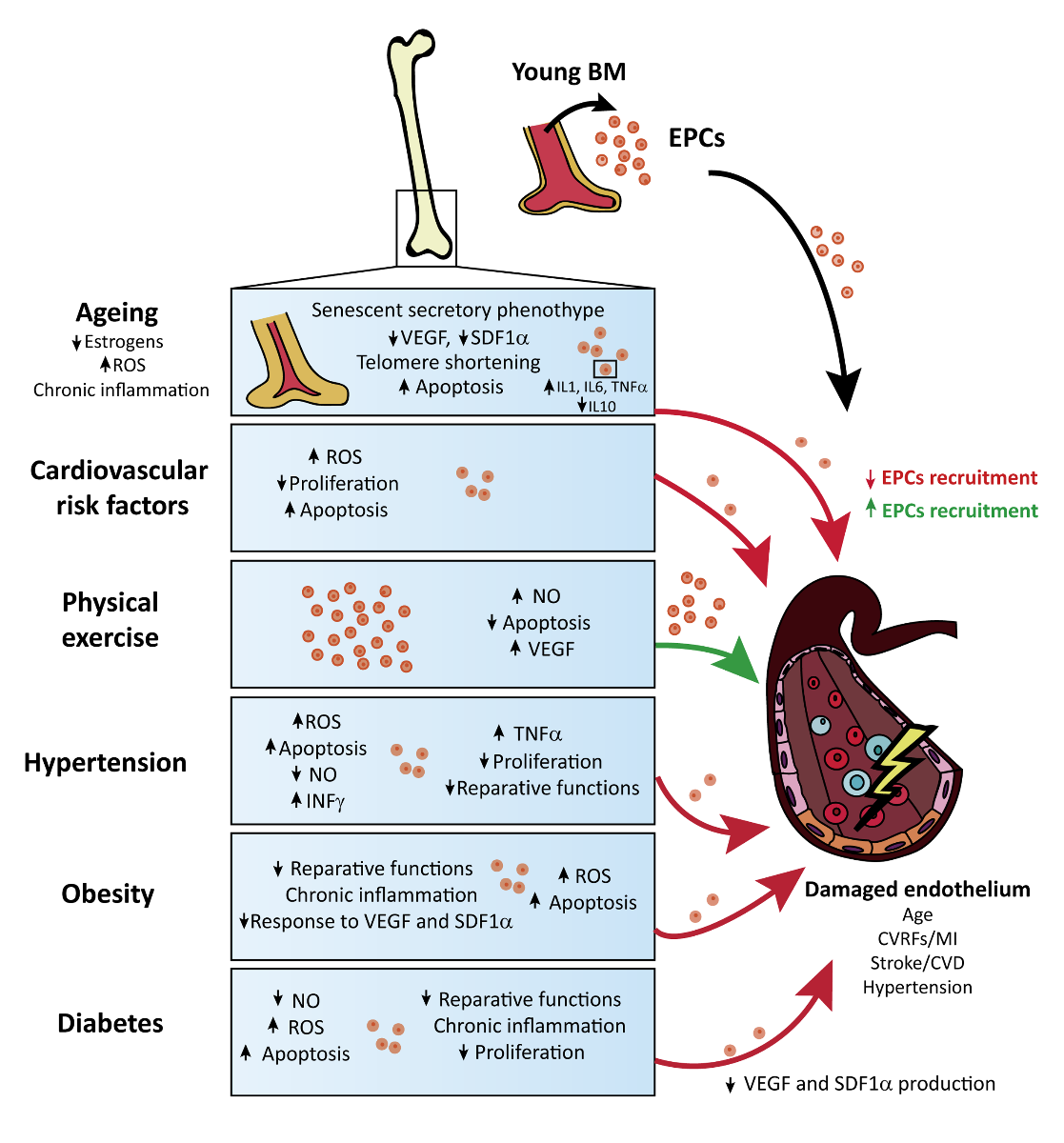
The Endothelium is one of the structures that is strongly affected by aging and by the comorbidities described above. Endothelial cells respond to physical/chemical stimulation by releasing appropriate substances to maintain vasomotor balance and vascular hemostasis. However, in pathological circumstances, the damaged endothelium needs to be repaired by the action of EPCs to recover its normal functions. Due to the important role of EPCs in vascular repair, specifically in CVD and stroke, the effect of various comorbidities on these stem cells will be reviewed in the following sections (see tables 1 to 5 and Figure 1). It is important to highlight that the different preclinical works described in the following sections have studied how each individual risk factor separately affects the properties of EPCs. However, following the Stroke Treatment Academic Industry Roundtable, or STAIR recommendations for stroke and CVD (Fisher et al., 2009; Kahle et al., 2012), the influence of several of the aforementioned risk factors on EPCs should be analyzed in the same animal model to increase the translation from bench to bed side and increase the potential reparative effects of these stem cells in comorbid patients.
In a new window | Download PPT
Figure 1: Schematic representation of the reported effects of comorbid conditions on EPC function and endothelial health in the context of cardiovascular disease.
3.1. Aging
Aging is a complex and multifactorial process that is determined to a large extent by genetics and influenced by a wide range of environmental factors. One of the effects of aging is the structural and functional decline of the cardiovascular system, which is primarily attributable to the accumulation of senescent cells within the vascular wall. In young people, after endothelial damage, BM-derived EPCs proliferate, enter the circulation and migrate to the site of injury, improving endothelial function. In elderly patients, however, there are two main types of age-related processes that impair vascular repair. The first process is the alteration of the microenvironment and structure of the BM, which is the main niche of EPCs, and the second process is a direct effect of aging on EPCs (Rurali et al., 2016).
Regarding the effects of aging on BM structure, the most important factor is the progressive reduction of hematopoietic tissue mass (also called red or active BM, which contains the EPC populations) paralleled by an increase in adipose tissue mass (also called inactive or yellow BM because of its typical color due to fatty replacement) (Ricci et al., 1990). In fact, it has been demonstrated that BM cellularity is strictly dependent upon a patient’s age, and a 30% reduction in active BM mass in the iliac crest has been demonstrated at 70 years of age (Gilleece, 1993). However, BM stem cells are also strongly dependent upon extra-niche factors, such as hormonal levels (estrogens), reactive oxygen species (ROS) overproduction, or chronic inflammation. In women, menopause is one of the physiological mechanisms of senescence that is characterized by the reduction of estrogens. It has been shown that a reduction of estrogens in menopausal women leads to an increase in pro-inflammatory cytokines, such as IL-6, favoring the accumulation of adipose tissue in the BM (Pfeilschifter et al., 2002). Interestingly, type 2 diabetes may also cause BM niche dysfunction through the generation of advanced glycation end-products, which accumulate in the extracellular matrix and can suppress proliferation, induce apoptosis, and increase ROS production (Leslie et al., 2012).
Another age-related effect on BM cellularity is the reduction in tissue renewal and the switch to a senescent secretory phenotype resulting from the accumulation of senescent cells. The secretory content of EPCs is very important, for instance, after stroke or in vascular dementia. In both pathologies, but primarily in stroke, not only is there an increase in proliferation/migration and the incorporation of EPCs into the blood vessels at the site of injury, but it has also been shown that EPCs release protective cytokines and growth factors that can induce the self-repair of injured endothelial cells (Esquiva et al., 2018; Maki et al., 2018). Senescent cells, however, secrete biologically active molecules that can disrupt the normal tissue microenvironment and affect the behavior of neighboring cells. For example, aging results in an increase in pro-inflammatory cytokines (IL-1, IL6 and tumor necrosis factor-alpha (TNF-α), a decrease in anti-inflammatory cytokines, such as IL-10, and a decrease in hematopoietic growth factors. These changes finally converge into the disappearance of the red BM (Abdelmagid et al., 2015).
The second age-related process that impairs vascular repair is the direct effect of aging on EPCs due to mechanisms such as telomere shortening, an exaggerated production of inflammatory cytokines/ROS, increased apoptosis, and a reduction of EPC-stimulating factors. Telomeres are the regions at the ends of the chromosomes, and their main function is to protect the chromosome from degradation during DNA replication. With each cycle of cell division, the telomeres progressively shorten until they reach a critical length, below which senescence is achieved (Kushner et al., 2009). Interestingly, EPCs, like others stem cells, can elongate their telomeres by expressing the telomerase reverse transcriptase enzyme (TERT), but aging induces a decreased activity of this enzyme by increasing oxidative stress and decreasing the bioavailability of NO (Herrera et al., 2010). Another age-related factor that causes EPC senescence is low grade but chronic inflammation, which leads to an increase in cytokines such as TNF-α. This cytokine, in particular, can induce premature EPC senescence and apoptosis by acting on silent information regulator type-1 (SIRT1). In fact, SIRT1 inhibition reduces telomere length and increases the activity of the pro-apoptotic factor p53 (Du et al., 2014). Moreover, TNF-α reduces EPC proliferation by increasing the phosphorylation of p38 and, consequently, the activation of CREB and cyclin D1 (Seeger et al., 2005).
Aging is also associated with a pro-apoptotic EPC phenotype that is characterized by the decreased expression of key anti-apoptotic proteins and an enhanced susceptibility to pro-apoptotic factors. For example, it has been demonstrated that EPCs obtained from healthy aged men exhibit increased levels of active caspase-3, which is an important enzyme involved in apoptosis, when compared with EPCs from younger patients (Kushner et al., 2011).
Finally, as indicated throughout this review, after any kind of injury, such as CVD or stroke, there is an increase in the release of factors that promote both the proliferation and migration of EPCs to the site of injury. These factors include VEGF and SDF-1, which are produced throughout the stabilization of intracellular hypoxia-inducible factor 1-alpha (HIF-1α) in response to hypoxic conditions. In fact, one of the age-related effects resulting in decreased EPC recruitment is the increase in the degradation of HIF-1α, which indirectly reduces the levels of VEGF and SDF-1 (Chang et al., 2007). A summary of selected publications on aging and EPCs function can be found in Table 1.
Table 1. Most cited articles regarding the effects of aging on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Aging |
Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting |
Journal of the American College of Cardiology |
Preoperative values of EPCs in 43 to 80 years old patients with stable coronary artery disease undergoing CABG were lowered with increasing age, similar to the lowering of plasma VEGF levels. Despite a significant increase in EPCs and release of cytochemokines during CABG, age was a major limiting factor for mobilization of EPCs. |
Clinical research |
303 |
|
|
Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1 |
Circulation Research |
The age-related decline in EPC number and function can be restored |
Clinical research and Experimental research |
213 |
||
|
Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia |
Circulation |
Aging impairs EPC trafficking to sites of ischemia through a failure of aged tissues to normally stabilize the HIF1α induced by hypoxia in ischemic tissues. |
Clinical research and Experimental research |
145 |
||
|
Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men |
Journal of Applied Physiology |
Aging adversely affects EPC function in terms of migration and CFU number, but a 3-months aerobic exercise intervention improved EPC clonogenic and migratory capacity in middle-aged and older healthy men. |
Clinical research and Experimental research |
112 |
||
|
Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity |
Journal of Hypertension |
Estrogen prevented the onset of EPC senescence, probably through the stimulation of telomerase, and potentiated their proliferative |
Experimental research |
104 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with aging, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “aging”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cells (EPCs), Coronary Artery Bypass Grafting (CABG), Vascular Endothelial Growth Factor (VEGF), Insulin-like Growth Factor-1 (IGF-1), Hypoxia-Inducible Factor 1alpha (HIF1α), Colony Forming Units (CFU).
3.2. Cardiovascular risk factors
CVD is one of the leading causes of death in many parts of the world. CVD is also a recognized age-dependent condition whose incidence is increased due to a longer life expectancy and by the combination of several factors, known as cardiovascular risk factors (CVRFs), including tobacco use, physical inactivity, hypertension, elevated low-density lipoprotein cholesterol, atherosclerosis, and a cluster of interrelated metabolic risk factors. All these factors have been shown to produce endothelial damage, which could be resolved by the action of EPCs; however, unfortunately, CVRFs also produce EPC dysfunction. One of the consequences of smoking is a higher level of oxidative stress and, as previously mentioned, an increase in the production of ROS inhibits proliferation and mobilization of EPCs, and promotes the apoptosis of EPCs. In fact, it has been demonstrated that there is a 50% reduction in the number of EPCs in smokers compared to controls (Michaud et al., 2006).
Regarding another CVRF, physical inactivity, it has been shown that physical exercise results in increased NO production, which has been shown to be related to increased EPC mobilization. In a study evaluating the effect of physical exercise on EPCs from patients with stable coronary artery disease, a higher number of circulating EPCs and reduced apoptosis on day 28 of physical exercise was reported, compared with the first day. It was also demonstrated that physical exercise increased the number of EPCs in the BM and peripheral blood due to the upregulation of NO and VEGF (Laufs et al., 2004).
Hypercholesterolemia is a major risk factor for atherosclerosis, and both conditions appear to be significantly correlated with endothelial damage. It has been shown that patients with hypercholesterolemia with high levels of oxidized low-density lipoprotein (oxLDL) exhibit a reduction in the number of EPCs, as these oxLDL are uptaken by EPCs, which generates oxidative stress and can therefore lead to apoptosis of these stem cells (Tie et al., 2010).
3.3. Hypertension
Although hypertension is a CVRF, it is considered separately in this review because it is one of the most prevalent diseases worldwide and is a major risk factor for CVD and stroke. Hypertension, like the other CVRFs, causes damage to the vascular endothelium and requires the protective actions of EPCs. Unfortunately, there are many clinical studies demonstrating that hypertension is the most important independent predictor of EPC functional decline in patients with coronary artery diseases (Vasa et al., 2001; see Table 2). Hypertension has also been associated with alterations in the circulating levels of EPCs, the impairment of their angiogenic properties, and alterations in their gene expression and cellular life span. In an attempt to summarize the effect of hypertension on EPCs from all of these clinical studies, first, the EPC functional decline appears to occur earlier than the reduction in EPC quantity in patients with hypertension. Second, the reduction in both EPC count and EPC function can be restored with antihypertensive treatment. Third, different types of EPCs may be differentially affected in patients with hypertension, i.e., late EPCs undergo more significant declines in their proliferative activity compared with other types of EPCs. Finally, the quantitative and functional decline of EPCs become more pronounced with advanced stages of hypertension, which can be manifested as intractable high blood pressure, increased incidence of adverse vascular events, and severe organ damage (Luo et al., 2016).
Table 2. Most cited articles regarding the effects of hypertension on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Hypertension |
Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease |
Circulation research |
Number of isolated EPCs was reduced in CAD patients compared to healthy volunteers. These EPCs presented impaired migratory response. Number of (Atherosclerotic) risk factors correlated with the reduction of EPCs and inversely with the migratory response capacity. This decrease in EPC number and function may contribute to impaired vascularization in CAD patients. |
Clinical research |
1906 |
|
|
Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension |
Journal of hypertension |
EPCs from hypertensive patients and both spontaneously hypertensive and DOCA-SH rats showed accelerated senescence and lower telomerase activity. EPC senescence may affect vascularization processes in hypertensive patients. |
Experimental research and Clinical research |
191 |
||
|
Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction |
Hypertension |
The re-endothelialized area of the EPCs from prehypertensive/hypertensive patients was lower than healthy subjects. Senescence of EPCs in prehypertension/hypertension was increased whereas NO production was reduced. Reduced endothelial repair capacity of EPCs was related to accelerated senescence and impaired endothelial function. |
Clinical research |
133 |
||
|
Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension |
American Journal of Hypertension |
Candesartan, an ARB used as an antihypertensive drug: increased the number of EPCs, reduced their oxidative stress response and the expression of NADPH oxidase subunits in cardiovascular organs of spontaneously hypertensive rats, improving EPC dysfunction. |
Experimental research |
83 |
||
|
Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect |
Hypertension Research |
Number, colony formation and migration of EPCs was reduced in SHR-sp compared to controls. After administration of Losartan (ARB), an increase in the number of circulating EPCs and in their colony formation capacity was observed together with an inhibition of oxidation in SHR-sp. Antihypertensives can be useful to repair vascular injuries related to hypertension. |
Experimental research |
80 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with hypertension, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “hypertension”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cells (EPCs), Coronary Artery Disease (CAD), deoxycorticosterone acetate-salt hypertensive(DOCA-SH), Nitric Oxide (NO), Angiotensin II Receptor Blocker (ARB), Nicotinamid-Adenin-Dinucleotide-Phosphate Nicotinamide adenine dinucleotide phosphate(NADPH), Spontaneously Hypertensive Stroke Prone Rat (SHR-sp).
There are several possible molecular explanations for these effects of hypertension on EPCs, which are common to the other CVRFs. For example, hypertension is associated with an accumulation of ROS, which causes an increase in apoptosis and senescence of EPCs. ROS also reduce NO availability, causing a reduction in EPC mobilization. Hypertension leads to a chronic increase in inflammatory molecules, such as TNF-α and interferon gamma (INF-γ, leading to a decrease in EPC proliferation, migration, and reparative functions (Luo et al., 2016).
3.4. Obesity and diabetes
Obesity is a widespread, increasingly prevalent disease with a crucial role in the development of endothelial dysfunction. Obesity is also associated with several comorbidities, including chronic inflammation, lipid metabolic disorder, accelerated atherosclerosis, increased risk for thrombosis, hypertension, hyperinsulinemia, insulin resistance, and type 2 diabetes mellitus, all of which can trigger CVD and stroke.
Endothelial dysfunction caused by obesity also requires the actions of EPCs to replace and regenerate the body vasculature (see highlighted publications summarized in Table 3). However, as with the other comorbidities, obesity affects the reparative functions of EPCs. In fact, in an experimental model of murine obesity, a reduced number of circulating EPCs with impaired functions were observed (Tsai et al. 2012). These experimental data have also been confirmed clinically; for example, a reduced number of circulating EPCs were shown in obese men with metabolic syndrome (MS) compared with lean men without MS (Westerweel et al., 2008). In another clinical study performed on obese subjects without diabetes or CVD, patients not only exhibited a decreased number of circulating EPCs but also an impaired functionality of these stem cells, compared to control subjects (Jialal et al., 2010). However, if this comorbidity is treated, as was shown in an interesting clinical study performed by Heida and colleagues in 2010, the poor pro-angiogenic properties of EPCs isolated from obese patients can be recovered after 6 months of weight loss therapy (Heida et al., 2010).
Table 3. Most cited articles regarding the effects of Obesity on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Obesity |
Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction |
European heart journal |
Obesity is associated with decreased numbers of CPCs and increased IMT: Inverse correlation between BMI and waist circumference and CPCs was observed. IMT increased together with BMI and correlated inversely with the number of EPCs. After diet, there was an increase in EPCs that correlated with the decrease in BMI and with the increase in CPC. Diet and weight loss may contribute to regression of IMT. |
Clinical research and Experimental research |
82 |
|
|
Adiponectin promotes endothelial progenitor cell number and function |
FEBS letters |
Adiponectin is downregulated in obese subjects; EPC levels did not increase in adiponectin deficient mice in response to hindlimb ischemia. But adenovirus mediated delivery of adiponectin increased EPC levels in both WT and KO mice. Incubation of human blood mononuclear cells with adiponectin led to an increase in the number of EPCs. Adiponectin also induced EPC differentiation into network structures and served as chemoattractant in EPC migration assays. The deficiency of adiponectin may contribute to decreased levels of EPC in obese patients. |
Experimental research |
74 |
||
|
Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy |
European heart journal |
Circulating EPC levels were reduced in obese men compared to non-obese healthy controls. Monotreatment of statins vs combination with a cholesterol absorption inhibitor both increased EPC circulating levels compared to control subjects. Treatment could contribute to endothelial regeneration and protect against cardiovascular disease. |
Clinical research |
64 |
||
|
Influence of BMI on level of circulating progenitor cells |
Obesity
|
Obesity is associated with fivefold increased frequency of CPCs but the number of mature endothelial cells is unaffected by the condition. Obesity could promote mobilization of progenitor cells. |
Clinical research and Experimental research |
60 |
||
|
Leptin receptor and functional effects of leptin in human endothelial progenitor cells |
Atherosclerosis |
High concentrations of leptin inhibited human EPC migration but did not have any effect on EPC proliferation. Thus, leptin may affect EPC function both in physiological and hyperleptinemic conditions. |
Experimental research |
59 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with obesity, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “obesity”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Circulating Progenitor Cells (CPC), Intima Media Thickness (IMT), Body Mass Index (BMI), Endothelial Progenitor Cells (EPCs), Wild Type (WT), Knockout (KO).
There are many molecular mechanisms through which obesity impairs EPC functions. One of the main consequences of obesity is the induction of chronic inflammation, a process that is also involved in the production of oxidative stress in many tissues, including the endothelium and the BM. As explained above, both mechanisms are implicated in EPC apoptosis and senescence, for example, by altering the expression of various cyclins, which are important regulators of the cellular cycle (Tobler et al., 2010). EPCs from obese patients have been shown to exhibit a decreased response to growth factors, such as VEGF and SDF-1, and a reduced paracrine potency, as they release reduced amounts of pro-angiogenic molecules such as IL-8 and monocyte chemoattractant protein-1 (MCP-1). Such alterations are, in part, prevented by inhibiting the MAP-kinase pathway (Heida et al., 2010).
As mentioned repeatedly throughout this review, the reparative actions of EPCs are very important in pathologies such as CVD and stroke. A clinical study performed on patients with CVD with and without MS demonstrated that, although there was an increased number of EPCs in CVD patients with MS when compared with patients without MS, interestingly, increased oxidative DNA damage, decreased telomerase activity, and decreased telomere length, a marker of increased senescence in EPCs, were observed in CVD patients with MS in comparison with the CVD patients without MS (Satoh et al., 2008). In the stroke field, it has been demonstrated that an increase in circulating EPCs after acute ischemic stroke is associated with good functional outcomes and reduced infarcts, even if patients have large-artery atherosclerosis and small-vessel disease (Martí-Fábregas et al., 2013; Sobrino et al., 2007). In this context, data from our research on aged and obese rats subjected to cerebral ischemia suggest a reduction in the circulating levels of EPCs, poorer pro-angiogenic properties in vitro, a reduction in the plasma levels of SDF-1, a worse post-stroke angiogenesis, and a worse functional recovery compared to aged lean animals (Pradillo et al., unpublished).
Diabetes, which is another CVRF, is a disease with increasing incidence in all societies. In most cases, diabetes develops as a consequence of obesity or MS. Diabetes is a complex metabolic disorder that is characterized by impaired glucose metabolism with hyperglycemia and alterations in the induction of endothelial damage with micro- or macro-vascular consequences, such as retinopathy, nephropathy, and accelerated atherosclerosis. A decrease in NO production by eNOS plays a critical role in the development and progression of endothelial damage and atherosclerosis in diabetes. Clinical studies have reported that EPCs are markedly reduced in patients with either type 1 or type 2 diabetes, and EPCs from diabetic patients also exhibit a reduced capacity to induce angiogenesis in vitro (Tepper et al., 2002). Likewise, impaired post-ischemic EPC mobilization in diabetic animals has been previously demonstrated (Fadini et al., 2006; Huang et al., 2010).
Several primary mechanisms have been proposed to explain the reduced number and dysfunction of EPCs that are associated with impaired glucose metabolism and diabetes. These mechanisms are inflammation, oxidative stress, reduced release of chemo-attracting factors, and reduction of NO. EPCs are first affected by diabetes in regard to their proliferation/mobilization from the BM. Both experimental and human studies have demonstrated eNOS dysfunction and reduced SDF-1α levels in the BM, both of which result in decreased EPC proliferation/mobilization from their niche (Fadini et al., 2013; Gallagher et al., 2007). The second level of by which diabetes affects EPC is by impairing EPC trafficking to the injured/ischemic area and their survival. In this setting, it has been shown that diabetes alters the cytokine gradient, reduces the VEGF and SDF-1α levels, decreases NO production, and increases the ROS levels and glycation end products, which are mechanisms involved in the diabetes-related EPC migration and survival effects. Finally, another level of influence by this disease is the process of EPC homing to the site of injury. For example, in this step, the local release of factors such as VEGF or SDF-1α is very important. In patients with diabetes, the high-glucose environment reduces the secretion levels of these factors from endothelial cells through mechanisms such as an increased degradation of the HIF-1α factor, which is the main transcription factor involved in the synthesis of the homing factors (Yiu and Tse, 2014). Table 4 highlights the findings of relevant publications demonstrating the negative effects of diabetes on EPCs.
Table 4. Most cited articles regarding the effects diabetes on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Diabetes |
Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures |
Circulation |
Type II diabetes may alter EPC biology: Proliferative capacity of diabetic EPCs was decreased compared to control subjects. Though EPCs had a normal adhesion to fibronectin, collagen and quiescent endothelial cells. They were also less likely to form tubules. |
Experimental research |
1153 |
|
|
Endothelial Progenitor Cell Dysfunction: A Novel Concept in the Pathogenesis of Vascular Complications of Type 1 Diabetes |
Diabetes |
The number of EPCs obtained from type 1 diabetic patients was lower than EPCs of control subjects, as well as their angiogenesis capacity. This dysfunction could contribute to the vascular complications in type 1 diabetes. |
Experimental Research |
672 |
||
|
Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus |
Journal of the American College of Cardiology |
The number of CPCs and EPCs from diabetic patients was reduced compared to healthy subjects. This reduction may be involved in the pathogenesis of peripheral vascular complications such as peripheral vascular disease. |
Experimental Research |
515 |
||
|
Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α |
Journal of Clinical investigation |
Diabetic mice showed impaired phosphorylation of BM eNOS which decreases the number of circulating EPCs. In a situation of hyperoxia the amounts of BM NO and circulating EPCs increased. Hyperoxia reverses the diabetic defect in EPC mobilization |
Experimental Research |
438 |
||
|
Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy |
Arteriosclerosis, Thrombosis and Vascular Biology |
EPC number is related to the severity to PAD complications in diabetic patients. Also, EPC function is altered in Diabetic patients with peripheral arterial disease. In this study patients with PAD showed a reduction in circulating EPCs versus non-PAD patients. Also, the clonogenic and adhesion capacity of these cells was lower in the diabetic PAD patients. |
Clinical research and Experimental research |
316 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with diabetes, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “diabetes”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cell (EPC), Circulating Progenitor Cells (CPC), Bone Marrow (BM), endothelial Nitric Oxide Synthase (eNOS), Nitric Oxide (NO), Peripheral Arterial Disease (PAD).
4. Conclusions and Future Perspectives.
As discussed, EPCs are progenitor cells found in adult subjects under physiological conditions, which respond to injury signals and hypoxic conditions by primarily contributing to vessel repair, angiogenesis, and vasculogenesis. Current literature highlights the importance of EPCs in maintaining a healthy vascular system, but has also shown strong evidence relating comorbid conditions such as hypertension, obesity and diabetes (strongly associated with CVDs), and aging, with EPCs decline and malfunction. These impaired abilities include reduced mobilization from the BM, smaller pools of circulating cells, decreased proliferation, altered endothelial functions, or declined paracrine potency. The good news is that some of these aberrant malfunctions of EPCs can be improved when the pathological conditions are reversed (e.g. with healthy diet, reduced glucose levels, or drug treatments such as statins), demonstrating that these cells might become future therapeutic targets to improve vascular health to prevent the manifestation CVDs. Finally, beyond the importance of those negative conditioning aspects in the context of CVDs, we should not forget the impact that they could also display in neurological diseases where the vascular crosstalk with other brain cells and the extracellular matrix is known to participate in disease progression or recovery.
Table 5. Most cited articles regarding the effects of other comorbidities on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Other comorbidities |
Circulating endothelial progenitor cells, vascular function, and cardiovascular risk |
New England Journal of Medicine
|
EPCs from subjects at high risk for cardiovascular events had higher rates of in vitro senescence than cells from subjects at low risk. Levels of EPCs may be a surrogate biologic marker for vascular function and cumulative cardiovascular risk. |
Clinical research and Experimental research |
2779 |
|
|
Selective Functional Exhaustion of Hematopoietic Progenitor Cells in the Bone Marrow of Patients With Postinfarction Heart Failure |
Journal of the American College of Cardiology |
Ischemic cardiomyopathy is associated with selective impairment of progenitor cell function in the bone marrow and in the peripheral blood, which may contribute to an unfavorable left ventricular remodeling process |
Clinical research and Experimental research |
189 |
||
|
p38 mitogen-activated protein kinase downregulates endothelial progenitor cells |
Circulation |
Incubation with glucose or TNF-α reduces the number of EPCs in vitro through the activation of p38 MAPK, while its inhibition by SB203580 (specific inhibitor of p38) prevents these negative effects. |
Clinical research and Experimental research |
182 |
||
|
Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction |
Clinical and Experimental Pharmacology and Physiology |
ox-LDL, a potential pro-atherosclerotic lipoprotein, accelerated the onset of EPC senescence, which may be related to telomerase inactivation, and lead to the impairment of their proliferative capacity and network formation in vitro. |
Experimental research |
169 |
||
|
Circulating CD34-positive cells provide an index of cerebrovascular function |
Circulation |
There is a strong inverse correlation between numbers of circulating EPCs and cerebral infarction, providing a potential marker of cerebrovascular function. |
Clinical research |
158 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with other comorbidites diferent to those shown in tables 1 to 4, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, “comorbidity”, and “comorbidites”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cell (EPC), Tumor Necrosis Factor alpha (TNF-α), p38 Mitogen-Activated Protein Kinase (p38 MAPK), oxidized Low-Density Lipoprotein (ox-LDL).
Acknowledgments
A. G. holds a predoctoral fellowship (FI17/00073) and A.R. is supported by the Miguel Servet program (CPII15/00003), both from Instituto de Salud Carlos III. This research has been funded with research grants from Fundació La Marató de TV3 (agreement 201731), SGR 2017/1427 from the Generalitat de Catalunya (AGAUR), S2017/BMD-3688 and M+Vision Fellowship from Comunidad de Madrid, and the PI16/00981 and PI17/01601, AC17/00004 and the INVICTUS RD16/0019/0021 and RD16/0019/0009 grants from the Instituto de Salud Carlos III, Spain, co-financed by the European Regional Development Fund. AC17/00004 is also part of the Euronanomed III funded Project MAGBBRIS.
References
Jesús M. Pradillo
1Departamento de Farmacología y Toxicología, Facultad de Medicina, Universidad Complutense de Madrid (UCM), Instituto de Investigación Hospital 12 de Octubre (i+12), Madrid, Spain.
Alba Grayston
2Neurovascular Research Laboratory and Neurology Department, Vall d’Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Violeta Medina-Alonso
1Departamento de Farmacología y Toxicología, Facultad de Medicina, Universidad Complutense de Madrid (UCM), Instituto de Investigación Hospital 12 de Octubre (i+12), Madrid, Spain.
Mercedes Arrúe
2Neurovascular Research Laboratory and Neurology Department, Vall d’Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Ignacio Lizasoain
1Departamento de Farmacología y Toxicología, Facultad de Medicina, Universidad Complutense de Madrid (UCM), Instituto de Investigación Hospital 12 de Octubre (i+12), Madrid, Spain.
Anna Rosell
2Neurovascular Research Laboratory and Neurology Department, Vall d’Hebron Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Corresponding author:
Anna Rosell
Email: anna.rosell@vhir.org

In a new window | Download PPT
Figure 1: Schematic representation of the reported effects of comorbid conditions on EPC function and endothelial health in the context of cardiovascular disease.
Table 1. Most cited articles regarding the effects of aging on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Aging |
Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting |
Journal of the American College of Cardiology |
Preoperative values of EPCs in 43 to 80 years old patients with stable coronary artery disease undergoing CABG were lowered with increasing age, similar to the lowering of plasma VEGF levels. Despite a significant increase in EPCs and release of cytochemokines during CABG, age was a major limiting factor for mobilization of EPCs. |
Clinical research |
303 |
Scheubel et al 2003 |
|
Age-dependent impairment of endothelial progenitor cells is corrected by growth hormone mediated increase of insulin-like growth factor-1 |
Circulation Research |
The age-related decline in EPC number and function can be restored |
Clinical research and Experimental research |
213 |
Thum et al 2007 |
|
|
Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia |
Circulation |
Aging impairs EPC trafficking to sites of ischemia through a failure of aged tissues to normally stabilize the HIF1α induced by hypoxia in ischemic tissues. |
Clinical research and Experimental research |
145 |
Chang et al 2007 |
|
|
Aging, exercise, and endothelial progenitor cell clonogenic and migratory capacity in men |
Journal of Applied Physiology |
Aging adversely affects EPC function in terms of migration and CFU number, but a 3-months aerobic exercise intervention improved EPC clonogenic and migratory capacity in middle-aged and older healthy men. |
Clinical research and Experimental research |
112 |
Hoetzer et al 2007 |
|
|
Estrogen reduces endothelial progenitor cell senescence through augmentation of telomerase activity |
Journal of Hypertension |
Estrogen prevented the onset of EPC senescence, probably through the stimulation of telomerase, and potentiated their proliferative |
Experimental research |
104 |
Imanishi et al 2005 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with aging, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “aging”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cells (EPCs), Coronary Artery Bypass Grafting (CABG), Vascular Endothelial Growth Factor (VEGF), Insulin-like Growth Factor-1 (IGF-1), Hypoxia-Inducible Factor 1alpha (HIF1α), Colony Forming Units (CFU).
Table 2. Most cited articles regarding the effects of hypertension on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Hypertension |
Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease |
Circulation research |
Number of isolated EPCs was reduced in CAD patients compared to healthy volunteers. These EPCs presented impaired migratory response. Number of (Atherosclerotic) risk factors correlated with the reduction of EPCs and inversely with the migratory response capacity. This decrease in EPC number and function may contribute to impaired vascularization in CAD patients. |
Clinical research |
1906 |
Vasa et al. 2001 |
|
Endothelial progenitor cell senescence is accelerated in both experimental hypertensive rats and patients with essential hypertension |
Journal of hypertension |
EPCs from hypertensive patients and both spontaneously hypertensive and DOCA-SH rats showed accelerated senescence and lower telomerase activity. EPC senescence may affect vascularization processes in hypertensive patients. |
Experimental research and Clinical research |
191 |
Imanishi et al 2005 |
|
|
Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction |
Hypertension |
The re-endothelialized area of the EPCs from prehypertensive/hypertensive patients was lower than healthy subjects. Senescence of EPCs in prehypertension/hypertension was increased whereas NO production was reduced. Reduced endothelial repair capacity of EPCs was related to accelerated senescence and impaired endothelial function. |
Clinical research |
133 |
Giannotti et al 2010 |
|
|
Effects of an ARB on endothelial progenitor cell function and cardiovascular oxidation in hypertension |
American Journal of Hypertension |
Candesartan, an ARB used as an antihypertensive drug: increased the number of EPCs, reduced their oxidative stress response and the expression of NADPH oxidase subunits in cardiovascular organs of spontaneously hypertensive rats, improving EPC dysfunction. |
Experimental research |
83 |
Yu et al 2008 |
|
|
Losartan improves the impaired function of endothelial progenitor cells in hypertension via an antioxidant effect |
Hypertension Research |
Number, colony formation and migration of EPCs was reduced in SHR-sp compared to controls. After administration of Losartan (ARB), an increase in the number of circulating EPCs and in their colony formation capacity was observed together with an inhibition of oxidation in SHR-sp. Antihypertensives can be useful to repair vascular injuries related to hypertension. |
Experimental research |
80 |
Yao et al 2007 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with hypertension, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “hypertension”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cells (EPCs), Coronary Artery Disease (CAD), deoxycorticosterone acetate-salt hypertensive(DOCA-SH), Nitric Oxide (NO), Angiotensin II Receptor Blocker (ARB), Nicotinamid-Adenin-Dinucleotide-Phosphate Nicotinamide adenine dinucleotide phosphate(NADPH), Spontaneously Hypertensive Stroke Prone Rat (SHR-sp).
Table 3. Most cited articles regarding the effects of Obesity on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Obesity |
Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction |
European heart journal |
Obesity is associated with decreased numbers of CPCs and increased IMT: Inverse correlation between BMI and waist circumference and CPCs was observed. IMT increased together with BMI and correlated inversely with the number of EPCs. After diet, there was an increase in EPCs that correlated with the decrease in BMI and with the increase in CPC. Diet and weight loss may contribute to regression of IMT. |
Clinical research and Experimental research |
82 |
Müller-Ehmsen et al 2008 |
|
Adiponectin promotes endothelial progenitor cell number and function |
FEBS letters |
Adiponectin is downregulated in obese subjects; EPC levels did not increase in adiponectin deficient mice in response to hindlimb ischemia. But adenovirus mediated delivery of adiponectin increased EPC levels in both WT and KO mice. Incubation of human blood mononuclear cells with adiponectin led to an increase in the number of EPCs. Adiponectin also induced EPC differentiation into network structures and served as chemoattractant in EPC migration assays. The deficiency of adiponectin may contribute to decreased levels of EPC in obese patients. |
Experimental research |
74 |
Shibata et al 2008 |
|
|
Endothelial progenitor cell levels in obese men with the metabolic syndrome and the effect of simvastatin monotherapy vs. simvastatin/ezetimibe combination therapy |
European heart journal |
Circulating EPC levels were reduced in obese men compared to non-obese healthy controls. Monotreatment of statins vs combination with a cholesterol absorption inhibitor both increased EPC circulating levels compared to control subjects. Treatment could contribute to endothelial regeneration and protect against cardiovascular disease. |
Clinical research |
64 |
Westerweel et al. 2008 |
|
|
Influence of BMI on level of circulating progenitor cells |
Obesity
|
Obesity is associated with fivefold increased frequency of CPCs but the number of mature endothelial cells is unaffected by the condition. Obesity could promote mobilization of progenitor cells. |
Clinical research and Experimental research |
60 |
Bellows et al 2011 |
|
|
Leptin receptor and functional effects of leptin in human endothelial progenitor cells |
Atherosclerosis |
High concentrations of leptin inhibited human EPC migration but did not have any effect on EPC proliferation. Thus, leptin may affect EPC function both in physiological and hyperleptinemic conditions. |
Experimental research |
59 |
Wolk et al 2005 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with obesity, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “obesity”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Circulating Progenitor Cells (CPC), Intima Media Thickness (IMT), Body Mass Index (BMI), Endothelial Progenitor Cells (EPCs), Wild Type (WT), Knockout (KO).
Table 4. Most cited articles regarding the effects diabetes on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Diabetes |
Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures |
Circulation |
Type II diabetes may alter EPC biology: Proliferative capacity of diabetic EPCs was decreased compared to control subjects. Though EPCs had a normal adhesion to fibronectin, collagen and quiescent endothelial cells. They were also less likely to form tubules. |
Experimental research |
1153 |
Tepper et al 2002 |
|
Endothelial Progenitor Cell Dysfunction: A Novel Concept in the Pathogenesis of Vascular Complications of Type 1 Diabetes |
Diabetes |
The number of EPCs obtained from type 1 diabetic patients was lower than EPCs of control subjects, as well as their angiogenesis capacity. This dysfunction could contribute to the vascular complications in type 1 diabetes. |
Experimental Research |
672 |
Loomans et al 2004 |
|
|
Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus |
Journal of the American College of Cardiology |
The number of CPCs and EPCs from diabetic patients was reduced compared to healthy subjects. This reduction may be involved in the pathogenesis of peripheral vascular complications such as peripheral vascular disease. |
Experimental Research |
515 |
Fadini et al 2005 |
|
|
Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α |
Journal of Clinical investigation |
Diabetic mice showed impaired phosphorylation of BM eNOS which decreases the number of circulating EPCs. In a situation of hyperoxia the amounts of BM NO and circulating EPCs increased. Hyperoxia reverses the diabetic defect in EPC mobilization |
Experimental Research |
438 |
Gallagher et al 2007 |
|
|
Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy |
Arteriosclerosis, Thrombosis and Vascular Biology |
EPC number is related to the severity to PAD complications in diabetic patients. Also, EPC function is altered in Diabetic patients with peripheral arterial disease. In this study patients with PAD showed a reduction in circulating EPCs versus non-PAD patients. Also, the clonogenic and adhesion capacity of these cells was lower in the diabetic PAD patients. |
Clinical research and Experimental research |
316 |
Fadini et al 2006 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with diabetes, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, and “diabetes”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers in the field of this comorbidity are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cell (EPC), Circulating Progenitor Cells (CPC), Bone Marrow (BM), endothelial Nitric Oxide Synthase (eNOS), Nitric Oxide (NO), Peripheral Arterial Disease (PAD).
Table 5. Most cited articles regarding the effects of other comorbidities on the biology of EPCs.
|
|
Title |
Journal |
Main Results |
Type of study |
Cit. |
Ref. |
|
Other comorbidities |
Circulating endothelial progenitor cells, vascular function, and cardiovascular risk |
New England Journal of Medicine
|
EPCs from subjects at high risk for cardiovascular events had higher rates of in vitro senescence than cells from subjects at low risk. Levels of EPCs may be a surrogate biologic marker for vascular function and cumulative cardiovascular risk. |
Clinical research and Experimental research |
2779 |
Hill et al 2003 |
|
Selective Functional Exhaustion of Hematopoietic Progenitor Cells in the Bone Marrow of Patients With Postinfarction Heart Failure |
Journal of the American College of Cardiology |
Ischemic cardiomyopathy is associated with selective impairment of progenitor cell function in the bone marrow and in the peripheral blood, which may contribute to an unfavorable left ventricular remodeling process |
Clinical research and Experimental research |
189 |
Kissel et al 2007 |
|
|
p38 mitogen-activated protein kinase downregulates endothelial progenitor cells |
Circulation |
Incubation with glucose or TNF-α reduces the number of EPCs in vitro through the activation of p38 MAPK, while its inhibition by SB203580 (specific inhibitor of p38) prevents these negative effects. |
Clinical research and Experimental research |
182 |
Seeger et al. 2005 |
|
|
Oxidized low-density lipoprotein induces endothelial progenitor cell senescence, leading to cellular dysfunction |
Clinical and Experimental Pharmacology and Physiology |
ox-LDL, a potential pro-atherosclerotic lipoprotein, accelerated the onset of EPC senescence, which may be related to telomerase inactivation, and lead to the impairment of their proliferative capacity and network formation in vitro. |
Experimental research |
169 |
Imanishi et al 2004 |
|
|
Circulating CD34-positive cells provide an index of cerebrovascular function |
Circulation |
There is a strong inverse correlation between numbers of circulating EPCs and cerebral infarction, providing a potential marker of cerebrovascular function. |
Clinical research |
158 |
Taguchi et al 2004 |
Foot note: In order to summarize the most relevant publications associating EPC levels and function with other comorbidites diferent to those shown in tables 1 to 4, a search on Scopus database was undertaken. Keywords included “endothelial progenitor cells”, “EPC”, “comorbidity”, and “comorbidites”. Only original articles were selected. Results were sorted according to the number of citations. The 5 most cited and relevant papers are listed, sorted from the most cited to the less according to Scopus database. Abbreviations: Cit (Citations), Ref (Reference), Endothelial Progenitor Cell (EPC), Tumor Necrosis Factor alpha (TNF-α), p38 Mitogen-Activated Protein Kinase (p38 MAPK), oxidized Low-Density Lipoprotein (ox-LDL).
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 10887 | 52 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA