Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Peripheral Mechanisms of Remote Ischemic Conditioning
Time:2019-05-03
Number:11780
Jiwon Yang1,2, Faariah Shakil1, Sunghee Cho1,3
Author Affiliations


- 1Burke Neurological Institute, White Plains, NY 10605.
- 2The Jackson Laboratory, Sacramento, CA 95838.
- 3Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY 10065.
Conditioning Medicine, 2019. 2(2):61-68.
Abstract
Ischemic conditioning induces an endogenous protective mechanism that allows organisms to develop resistance to subsequent insults. The conditioning effect occurs across organs and species. Recently, much attention has been given to remote ischemic limb conditioning due to its non-invasive nature and potential therapeutic applications. While tolerance is induced at the primary injury site (e.g. the heart in cardiac ischemia and the brain in stroke), the site of conditioning application is away from the target organ, suggesting the protective factors are extrinsic in nature rather than intrinsic. This review will focus on the peripheral factors that account for the induction of tolerance. Topics of particular interest are blood flow changes, peripheral neural pathways, humoral factors in circulation, and the peripheral immune system. This review will also discuss how conditioning may negatively affect metabolically compromised conditions, its optimal dose, and window for therapy development.
Keywords: remote limb conditioning; immune-mediated mechanism; ischemia/reperfusion injury
Abstract
Ischemic conditioning induces an endogenous protective mechanism that allows organisms to develop resistance to subsequent insults. The conditioning effect occurs across organs and species. Recently, much attention has been given to remote ischemic limb conditioning due to its non-invasive nature and potential therapeutic applications. While tolerance is induced at the primary injury site (e.g. the heart in cardiac ischemia and the brain in stroke), the site of conditioning application is away from the target organ, suggesting the protective factors are extrinsic in nature rather than intrinsic. This review will focus on the peripheral factors that account for the induction of tolerance. Topics of particular interest are blood flow changes, peripheral neural pathways, humoral factors in circulation, and the peripheral immune system. This review will also discuss how conditioning may negatively affect metabolically compromised conditions, its optimal dose, and window for therapy development.
Keywords: remote limb conditioning; immune-mediated mechanism; ischemia/reperfusion injury
Introduction
Ischemic conditioning is an endogenous protective mechanism that protects various organs and tissues by application of sub-lethal stimuli (Dirnagl et al., 2009). Several different conditioning paradigms have been reported based on the type of conditioning stimulus, including ischemia/hypoxia and immunological or pharmacological agents. Depending on the time of the application in relation to the occurrence of the full ischemic insults, the conditioning paradigm is considered as pre (before), per (during), or post (after) ischemic conditioning. The sub-lethal noxious stimuli may be applied directly onto the targeted organs to induce tolerance, or, alternatively, it may be applied away from the target organs. This is referred to as remote conditioning and induces cross-organ tolerance. Remote ischemic limb conditioning (RLC) against myocardial infarction showed a protective effect in rabbits and pigs (Birnbaum et al., 1997; Kharbanda et al., 2002). Furthermore, preclinical studies and clinical trials indicated tolerance induction across organs, including heart, brain, retina, and kidney (White et al., 2015; Zarbock and Kellum, 2016; England et al., 2017; Chong et al., 2018; Gidday, 2018).
Among conditioning paradigms, RLC has been given much more attention due to its non-invasive nature and feasibility in clinical application. Clinical trials of RLC established RLC as safe in patients with severe carotid artery stenosis, subarachnoid hemorrhage, and ischemic stroke (Gonzalez et al., 2014; England et al., 2017; Zhao et al., 2017). RLC also showed favorable effects in infarct reduction after cardiac ischemia and elective coronary artery bypass grafting surgery (Hausenloy et al., 2007; Prunier et al., 2014). The majority of early preclinical RLC studies were done in a pre-RLC setting, meaning RLC was performed before the disease as a preventative application. Because of the clinical applicability, however, the focus has been moved to post-RLC, a method of applying RLC after disease onset. Most of the clinical and preclinical post-RLC studies have performed RLC acutely after disease onset and those studies showed protective effects of RLC (Hoda et al., 2012; Hoda et al., 2014; Kono et al., 2014; Khan et al., 2018). However, a number of recent clinical trials and preclinical studies are trying to expand the therapeutic window, from days to weeks after the onset of the condition or disease, to find the optimal therapeutic time window. One “dose” of RLC in ongoing clinical trials mostly consists of 3-5 cycles of 5-10 min inflation and deflation, regardless of the type of disease (ClinicalTrials.gov identifier NCT02779712, NCT03794947, NCT03233919, NCT03566654 etc.). Most of the preclinical studies also adapted the above-mentioned intervention paradigm and confirmed that this “one dose” shows a protective effect in animal models (Hoda et al., 2014; Kim et al., 2014; Khan et al., 2018). However, the optimal dose and time point of RLC have not been fully studied yet. A detailed discussion about the RLC paradigm and dose are discussed in “Optimal therapeutic window and dose of conditioning” section in this review.
The underlying mechanism(s) for the tolerance induction by RLC may be multiple. Because RLC is performed remotely on the limb and the tolerance is achieved in the tissue/organ away from the site of application, underlying events that induce tolerance are likely derived from the periphery. In this review, we will evaluate potential mediators for RLC-induced inter-organ protection and how peripheral conditions influence RLC outcome for potential therapy development.
Mediators for RLC tolerance induction
RLC initiates a cascade of complex mechanisms that ultimately leads to the protection of a remote organ/tissue. Studies have reported several different mechanisms underlying RLC protection, mostly under cardiac and cerebral ischemic conditions. Since RLC is an inter-organ protective mechanism, the induction of tolerance likely involves several extrinsic factors including changes in blood flow, neural pathways, circulating factors, and alterations in peripheral immunity (Figure 1).
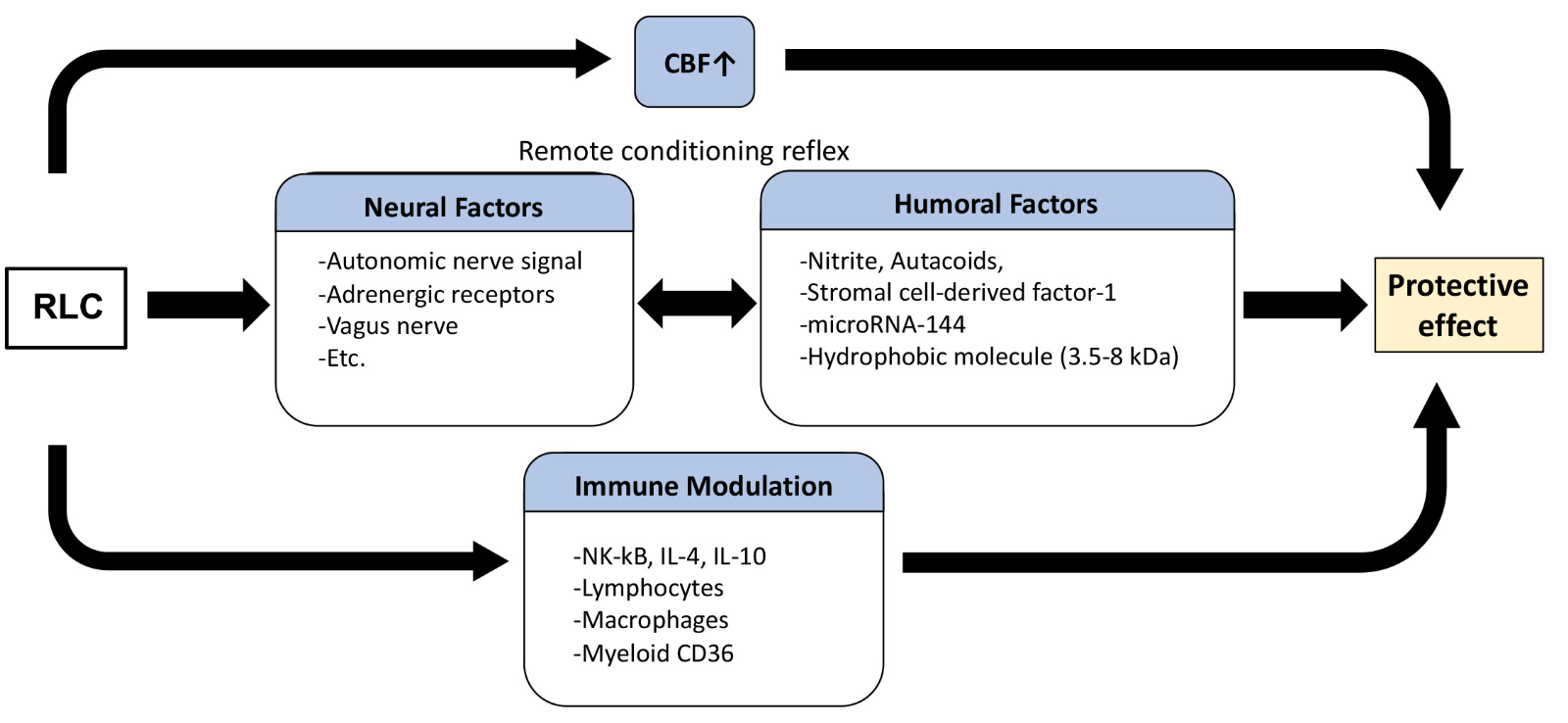
In a new window | Download PPT
Figure 1: Proposed protective mechanism of remote limb conditioning. RLC induces a host of changes that converge into the induction of tolerance. The peripheral factors triggered by RLC include changes in blood flow, neuronal and humoral factors, and immunity. These factors exert their effects either singly or interactively.
Blood flow
Many studies reported the effect of RLC in enhancing blood flow. Nitrite, a well-known vasodilator, has been considered a potential mediator of the RLC effect (Rassaf et al., 2014; Hess et al., 2016; Khan et al., 2018). RLC increases microcirculation and oxygen saturation in the surgical flaps, which ultimately leads to improved flap survival (Kolbenschlag et al., 2016). Microcirculation and portal venous flow and pressure are also increased by RLC in a rat model of arterialized orthotopic liver transplantation (Czigany et al., 2018). A positive effect of RLC on coronary circulation has been reported in a number of studies. Clinical studies showed that RLC intervention increased coronary microcirculation and coronary flow velocity in healthy subjects and in heart failure patients (Kono et al., 2014; Santillo et al., 2018). Similarly, RLC significantly increased coronary flow and left ventricular pressure in Lewis rats (Zhou et al., 2007). In cerebral ischemia, a significant increase in cerebral blood flow was reported in mouse models of stroke (Hoyte et al., 2006; Hoda et al., 2012; Hoda et al., 2014). RLC also increased cerebral perfusion in patients with symptomatic atherosclerotic intracranial arterial stenosis (Meng et al., 2012). While evidence strongly indicates that RLC has a role in increasing blood flow, it remains to be addressed whether RLC’s effect on blood flow would be sustained in metabolically compromised conditions associated with vascular dysfunction.
Neural factors
Among multiple events underlying RLC-induced tolerance, studies indicated the transduction of protective signals from the site of RLC to the target organ/tissue through neural pathways. In preclinical studies of myocardial ischemia, the cardioprotective effect of RLC in a mesenteric artery occlusion rat model involves autonomic nerve signals, as the cardioprotection was abolished by hexamethonium, a ganglion blocker (Gho et al., 1996; Schoemaker and van Heijningen, 2000). Similarly, reversal of RLC protection by blocking autonomic nerve stimulation was also reported by ganglion blockade in humans (Loukogeorgakis et al., 2005), suggesting the role of neural pathways in RLC protection. The blockade of the adrenergic receptors α1 by prazosin or β by timolol and propranolol, as well as inhibition of the muscarinic receptor by atropine, attenuated RLC protection (Southerland et al., 2007; Mastitskaya et al., 2012; Donato et al., 2013). Surgical resection such as vagotomy also abolished RLC-mediated protection in a rat and rabbit model of myocardial ischemia (Basalay et al., 2012; Donato et al., 2013). Inversely, the activation of the sensory system showed protective effects similar to that of RLC application in heart ischemia. Neural signals were transferred from the remote site to the heart in response to sensory fiber activation induced by capsaicin, bradykinin, adenosine, and electric nerve stimulation (Schoemaker and van Heijningen, 2000; Jones et al., 2009; Steensrud et al., 2010; Redington et al., 2012; Gross et al., 2013; Johnsen et al., 2014; Merlocco et al., 2014). Moreover, transection of the spinal cord at T7-T10 abolished RLC protection (Jones et al., 2009; Donato et al., 2013) and spinal stimulation at C8-T2 reduced injury size (Southerland et al., 2007). These studies collectively suggest the involvement of neural pathways in the induction of tolerance by RLC.
Humoral factors
Early RLC studies showed the involvement of circulating factors in RLC-mediated protection. One such evidence was that a blood transfusion from a RLC rabbit to a control rabbit reduced myocardial infarct (Dickson et al., 1999). Similarly, RLC performed in recipient pigs reduced myocardial infarction after heart transplantation (Konstantinov et al., 2005), suggesting the presence of factors in the circulation may underlie the RLC effect. Other experimental evidence comes from a parabiotic study. One parabiont mouse received intracerebral hemorrhage while the other mouse received RLC without injury. The study showed that RLC application on the uninjured pair-mate accelerated hematoma clearance of the injured pair-mate (Vaibhav et al., 2018). Additionally, cardiac function in a Langendorff system, a method using isolated perfused hearts from warm-blooded animals according to Langendorff (Broadley, 1979), was improved by serum from humans subjected to RLC providing further evidence for the involvement of humoral factors in circulation for RLC protection (Heinen et al., 2018) .
While the exact nature of RLC-induced mediators in circulation has not been identified, a study showed that RLC results in changes in plasma proteomics in humans (Hepponstall et al., 2012). In preclinical studies using genetic and pharmacological approaches, circulating nitrite has been suggested as a key mediator of RLC, since the absence of nitric oxide synthase abolished RLC protection (Rassaf et al., 2014; Hess et al., 2016; Khan et al., 2018). In addition, autacoids, such as bradykinin, adenosine, opioids, and angiotensin, (Schoemaker and van Heijningen, 2000; Liem et al., 2002; Sharma et al., 2015; Randhawa and Jaggi, 2016, 2017) increased antioxidant capacity (Morihira et al., 2006; Dong et al., 2010). Stromal cell-derived factor-1 (Davidson et al., 2013), microRNA-144 (Li et al., 2014; Przyklenk, 2014), and a hydrophobic molecule with a molecular mass between 3.5 and 8 kDa (Lang et al., 2006; Serejo et al., 2007), were also suggested as RLC mediators. Thus, a wealth of literature indicates the involvement of multiple humoral factors that induce RLC protection.
The connection of neural and humoral pathway
As RLC mechanism involves a series of cascades, a concept of “remote conditioning reflex” has been suggested that links the neural pathway and humoral system to the RLC effect (Gourine et al., 2010). In a mouse model of myocardial infarction, RLC was performed while interrupting a humoral pathway by the occlusion of a femoral vein or blocking neuronal pathways by femoral and/or sciatic nerve resection. The blocking of humoral and nerve pathways completely abolished the RLC-induced protective effect, while the nerve resection partially abolished the protection (Lim et al., 2010). Another experiment showed the involvement of the vagus-spleen axis in RLC protection in pig and rat models of coronary occlusion, as RLC protection was abolished through vagotomy and splenectomy (Lieder et al., 2018), supporting the involvement of both neural and humoral pathways in RLC protection. Clinical studies also showed the efficacy of RLC by treating the Langendorff model of rabbit hearts with blood dialysates collected from humans before and after the RLC (Jensen et al., 2012). The study found that RLC dialysates from healthy humans and diabetics without neuropathy showed protection, while those from subjects with diabetes with neuropathy failed to protect cardiac function in rabbits (Jensen et al., 2012). While these findings indicate that multiple RLC-released circulating factors in conjunction with an intact neural pathway account for RLC protection, the interaction can be disrupted in comorbid conditions. Future investigations should aim to understand neural and humoral interactions in normal and comorbid conditions and their protective effects in RLC.
Immune-mediated responses
RLC has been shown to modulate systemic inflammation by altering several inflammatory pathways. For instance, RLC significantly decreased LPS-induced systemic inflammation by altering the NF-κB signaling pathway (Kim et al., 2014). RLC was shown to increase IL-4 and IL-10 anti-inflammatory cytokines in a lung injury model (Zhou et al., 2017). A microarray of human blood samples showed changes in inflammatory gene expression by reducing expression of pro-inflammatory genes in leukocytes, leukocyte chemotaxis, apoptosis, and innate immune system signaling pathways (Konstantinov et al., 2004).
In addition to the aforementioned changes in inflammation-related cytokines and chemokines, RLC-induced changes occur at the immune cell level. It has been shown that RLC induced changes in immune cell composition in circulation and in the spleen, an immediate reservoir of immune cells upon injury (Swirski et al., 2009). In naïve animals, RLC increased the total number of lymphocytes in the spleen and prevented T cell release into the blood from the spleen while increasing B cell release into the blood (Chen et al., 2018). In cerebral ischemia, RLC abolished stroke-induced spleen shrinkage, blocked splenic immune cell release, including T cell, B cell, and NK cell, into circulation, and reduced infiltration of those cells into the stroked brain (Chen et al., 2018). These results suggest that RLC modulates constituents of splenic immune cells in naïve and injured conditions and also their release and trafficking upon injury. In line with this result, Liu et al. (2018) also reported that RLC causes CD4 and CD8 lymphopenia in the spleen and circulation after ischemic stroke (Liu et al., 2018). RLC reduced LPS-induced neutrophil infiltration in the liver that led to decreased systemic inflammation (Kim et al., 2014) and reduced neutrophil adhesion, phagocytic activity, and apoptosis in healthy subjects (Shimizu et al., 2010).
Monocytes/macrophages have been implicated in stroke-induced inflammation and injury. RLC increased non-inflammatory resident monocytes without changing inflammatory monocytes in a rat model of ischemic stroke (Liu et al., 2016). In hemorrhagic stroke, RLC polarized macrophages to the anti-inflammatory phenotype and increased hematoma clearance in an AMPKα1-dependent manner (Vaibhav et al., 2018). Using bone marrow chimera, the same study showed a role for scavenger receptor CD36 in RLC protective effect, since the absence of CD36 in monocytes/macrophages abolished RLC-induced hematoma clearance, while transplant wild-type bone marrow into CD36 knock-out recipients still showed the RLC protective effect (Vaibhav et al., 2018). This suggests that myeloid CD36 may be a critical mediator of the RLC protective effect. While these studies indicate that RLC shifts the brain to a less inflammatory state, it is not known whether the RLC-induced shift in the brain is due to reduced inflammation or enhanced inflammation resolution. However, the mechanisms by which RLC modulates cellular constituents in the periphery that potentially accelerate resolution in the injured brain remain to be identified.
Exercise and conditioning
Exercise is overall beneficial to health, as it protects a host from metabolic dysfunction such as type 2 diabetes, stroke, and coronary artery disease (Shinton and Sagar, 1993; Yadav, 2007; Colberg et al., 2016). Evidence suggests that RLC improves exercise performance by directly affecting limbs (especially lower limbs) or by improving functions of a remote organ, particularly the heart. Studies performed on cyclists found that RLC on the legs improved maximal oxygen uptake, exercise performance, swim time, and running trial (de Groot et al., 2010; Crisafulli et al., 2011; Jean-St-Michel et al., 2011; Bailey et al., 2012). The underlying mechanism of exercise performance improvement by RLC includes vasodilatation in skeletal muscle through activation of vascular smooth muscle KATP channels and enhancement of adenosine release (Duncker et al., 1993; Joyner and Proctor, 1999), preservation of blood flow in lower extremities (Bailey et al., 2012), an increase in coronary blood flow (Shimizu et al., 2007; Zhou et al., 2007), and extracellular matrix peptides LG3 (Salmeron et al., 2018).
Exercise also changes circulating immune cells (Tipton, 2014). For instance, exercise increases the number of lymphocytes and their subsets, including NK cells and T cells, in the blood (Gleeson and Bishop, 2005), and also regulates circulating monocytes (Lancaster and Febbraio, 2014). RLC alters immune cell circulation in a similar manner as exercise. Thus, a common mechanism shared by RLC and exercise suggests that exercise can be considered a form of conditioning stimuli. A critical advantage of RLC, however, lies in its applicability, as it can be performed on individuals who are unable to exercise.
Negative conditioning
Despite favorable outcomes in animal models, results from clinical trials of conditioning have been inconclusive and afflicted by heterogeneity. Although numerous studies showed the protective effect of RLC, others reported that RLC did not have an effect on average running or sprint speed, oxygen uptake, aerobic energy cost, maximal heart rate, and blood pressure (de Groot et al., 2010; Patterson et al., 2015; Tocco et al., 2015). Moreover, a number of studies also reported negative results of RLC in different disease settings (Secher et al., 2014; Baranyai et al., 2015; Kierulf-Lassen et al., 2015; Sloth et al., 2015). These studies have shown that ischemic conditioning-mediated protection is abrogated in individuals with comorbidities, such as hypercholesterolemia, diabetes, and hypertensive conditions (Szilvassy et al., 1995; Tosaki et al., 1996). In a preclinical study with normoglycemic and acute hyperglycemic Wistar rats, the cardioprotective effect of RLC was abolished in the hyperglycemic group (Baranyai et al., 2015). In post hoc subgroup analysis of ST-elevation myocardial infarction patients, RLC intervention was less effective for smokers as compared to non-smokers, but other comorbidities such as lipid level, glucose level, and medication use did not affect RLC protection (Sloth et al., 2015). Based on these findings, it is reasonable to predict that comorbidities influence the outcome of RLC effect or negatively influence conditioning-mediated protection (Figure 2). Comparison studies between normal and comorbid animals can identify the inconsistencies of conditioning effects and facilitate the design of clinical trials based on comorbidities.
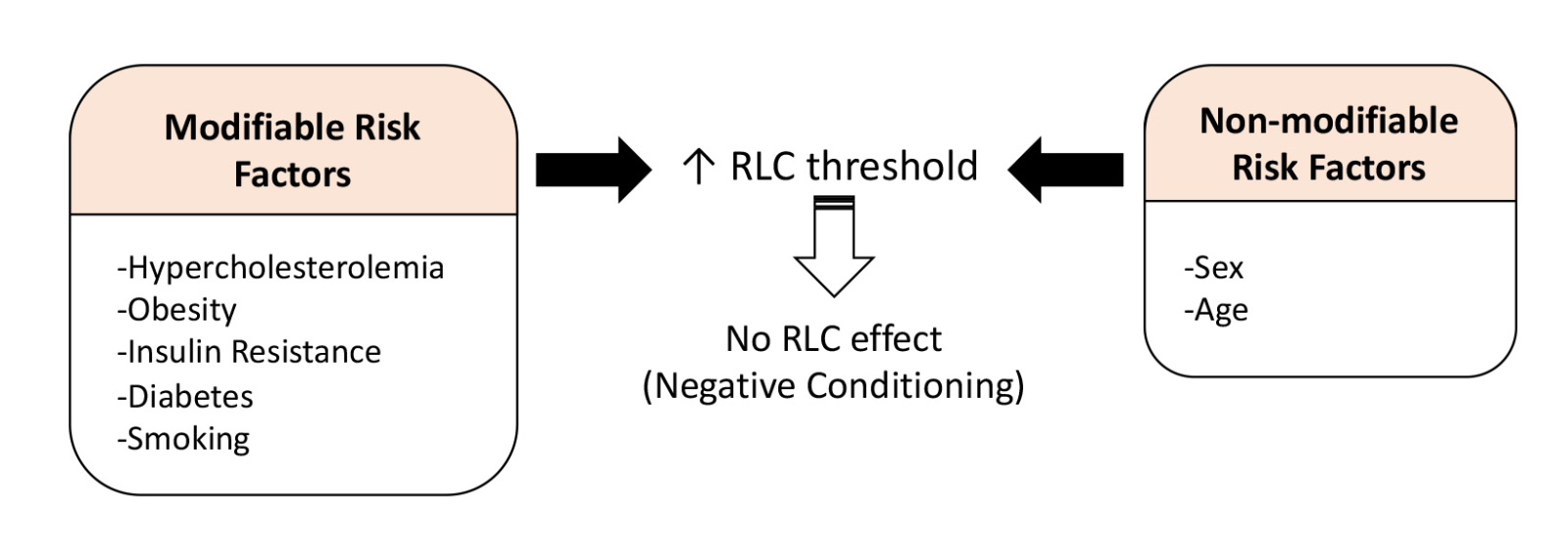
In a new window | Download PPT
Figure 2: Effect of vascular risk factors on RLC-induced protection. Modifiable risk factors associated with metabolic dysfunctions and smoking, as well as non-modifiable risk factors (sex and age), attenuate or abolish the conditioning effect. The effect may be mediated through increasing thresholds of RLC, which requires validation of optimal doses in metabolically compromised conditions.
Similar to the negative effect of RLC in individuals with metabolic dysfunctions, studies also suggest that age and sex, non-modifiable risk factors, affect the RLC-mediated protective effect. In a study using a Langendorff system with rat hearts, RLC plasma obtained from young male volunteers reduced isolated heart infarct size from both young and aged rats (Heinen et al., 2018). On the other hand, RLC plasma from female volunteers failed to reduce infarction regardless of the donor age (Heinen et al., 2018). Another animal study reported the effect of age by showing the absence of RLC-mediated cardioprotection in aged (22-24 months) male rats (Behmenburg et al., 2017). Other preclinical studies claimed that age and comorbid conditions raise the threshold for RLC-induced protection because those conditions need more robust conditioning signals (Boengler et al., 2009; Ferdinandy et al., 2014) (Figure 2). Further details of the effect of risk factors, comorbidities, co-medications, and anesthetics in RLC, and the challenge of RLC are discussed elsewhere (Ferdinandy et al., 2014; McCafferty et al., 2014; Wider and Przyklenk, 2014; Epps and Smart, 2016). Although not fully established, the means by which comorbidities and/or medications influence the protective effect of RLC remains to be fully established in order to develop RLC as a reliable therapeutic for patients.
Optimal therapeutic window and dose of conditioning
One challenge regarding RLC is the determination of proper dose and therapeutic window for a maximal protective effect in patients. The most commonly used RLC paradigm is 3-5 cycles of 5 min inflation and 5 min deflation. Meta-analysis of RLC in myocardial infarct patients showed the maximal protective effect when RLC was applied for at least 3 cycles and ≥ 30 min duration (Man et al., 2017). An interesting result from the study was that an increased dose of RLC often does not correlate with a better outcome. In fact, while 4-6 cycles of RLC showed significant protection after cardiac ischemia, 8 cycles did not show further protection in a mouse model (Johnsen et al., 2016). Two min and 5 min inflation/deflation showed the same protective effect, while prolonged 10 min inflation/deflation cycle abolished the protection (Johnsen et al., 2016). On the other hand, other studies reported that repeated RLC showed more consistent beneficial effect with dose-dependent improvement (Wei et al., 2011; Jones et al., 2014; Yamaguchi et al., 2015). We observed, however, that repeated RLC (RLC every 24 hours up to 3D or 7D) did not show an additional effect on peripheral monocyte subset change as compared to a single conditioning (Yang et al., 2018). Another preclinical study in a vascular cognitive impairment and dementia model showed that daily RLC up to 1 month is equally effective as daily RLC up to 4 months (Khan et al., 2018), suggesting dose-dependency of RLC occurs in a limited frame. Different paradigms of RLC also have been employed in phase II clinical trials to establish the optimal dose of RLC. ReCAST-2 study (ClinicalTrials.gov identifier NCT02779712) performed 3 different RLC paradigms: a single dose of 4 cycles of 5 min inflation/deflation, an additional repeated dose of 4 cycles of 5 min inflation/deflation 1 hour after the initial dose, and repeated doses at one-hour intervals applied daily for 4 days. Regarding the site of RLC, it has been reported no significant difference in RLC effect when it was applied on either arm or thigh (Dezfulian et al., 2017). It was also reported one vs. two hind-limb conditioning showed an equal protective effect (Johnsen et al., 2016).
The time of RLC application is an additional variable that requires consideration. Most of the preclinical studies in myocardial infarct models performed RLC during ischemia or right after reperfusion. In our mouse model of ischemic stroke, we also observed a protective effect when RLC was applied 2h after stroke (Yang et al., 2018). Therapeutic phases for RLC protection occur at acute phases (within 2 hours) and delayed phases (up to 3 days) (Kuzuya et al., 1993; Marber et al., 1993; Guo et al., 1998). A difference in outcome between early application of RLC at 12-24h after stroke and delayed application at 120h after stroke in an ischemic stroke model has been reported (Doeppner et al., 2018). In this study, RLC performed at 12h and 24h significantly reduced the infarct volume, while 120h post-RLC did not. However, the latter delayed RLC group showed improved neurological recovery up to 84 days, indicating that RLC can be protective across acute pathology and recovery depending on the time of RLC application. While therapeutic windows based on animal studies are difficult to translate to humans, few clinical trials have applied delayed RLC. CORIC-MI study (ClinicalTrials.gov identifier NCT03233919) is designed to apply RLC immediately after or 2-28 days post myocardial infarction. Once the ongoing clinical trials are completed, there will be a better understanding of optimal dose and therapeutic time window for RLC that can be applied to patients.
Summary and conclusion
Progression of injury development in cardio and cerebrovascular diseases is profoundly influenced by peripheral circulating factors, immune cells, and blood flow. It is thus reasonable to modify peripheral systems to alter injury development and subsequent repair processes. One such manipulation is RLC, which triggers changes in the peripheral system to induce an endogenous tolerance mechanism in target organs. Studies in multiple species have shown that ischemic conditioning confers resistance to subsequent insults, an effect that is neither organ- nor tissue-specific. Unlike the direct application of conditioning to the tissue at risk, RLC can be applied remotely from the primary injury site. RLC is advantageous because it is an effective and non-invasive way to remotely induce cross-tolerance in multiple organs. Emerging evidence indicates that it can be applied after the primary insult, thereby providing therapeutic applicability in a clinical setting. Since RLC is applied away from protected organs (i.e., brain or heart), a host of RLC-induced peripheral changes including blood flow, neural pathways, circulating factors, and immune mediators are implicated as underlying events for tolerance induction. A remaining challenge of RLC is understanding adaptive changes in the peripheral system in metabolically compromised conditions with vascular dysfunction, which attenuate or nullify the effect of RLC. Additionally, optimization of dose and time window of post-stroke RLC in normal and comorbid conditions may be necessary to develop effective RLC strategies in future clinical trials.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
This work was supported by NIH grants HL082511, NS095359, and NS077897 (SC), Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education 2013R1A6A3A03059436 (JY), and American Heart Association Postdoctoral Fellowship 15POST25680020 (JY).
References
Jiwon Yang1,2
1Burke Neurological Institute, White Plains, NY 10605. 2The Jackson Laboratory, Sacramento, CA 95838.
Faariah Shakil1
1Burke Neurological Institute, White Plains, NY 10605.
Sunghee Cho1,3
1Burke Neurological Institute, White Plains, NY 10605. 3Feil Family Brain and Mind Research Institute, Weill Cornell Medicine, New York, NY 10065.
Corresponding author:
Sunghee Cho
Email: suc2002@med.cornell.edu

In a new window | Download PPT
Figure 1: Proposed protective mechanism of remote limb conditioning. RLC induces a host of changes that converge into the induction of tolerance. The peripheral factors triggered by RLC include changes in blood flow, neuronal and humoral factors, and immunity. These factors exert their effects either singly or interactively.

In a new window | Download PPT
Figure 2: Effect of vascular risk factors on RLC-induced protection. Modifiable risk factors associated with metabolic dysfunctions and smoking, as well as non-modifiable risk factors (sex and age), attenuate or abolish the conditioning effect. The effect may be mediated through increasing thresholds of RLC, which requires validation of optimal doses in metabolically compromised conditions.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 11780 | 35 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA