Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Detraining ablates exercise-induced modulation of the immune system after stroke
Time:2019-05-03
Number:9301
Katherine Poinsatte1, Sterling B. Ortega1, Uma M. Selvaraj1, Anouk J. M. Meeuwissen1, Xiangmei Kong1, Erik J. Plautz1, Nancy L. Monson1, Rong Zhang1,2, Ann M. Stowe1,3
Author Affiliations


- 1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
- 2Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX, USA.
- 3Department of Neurology, University of Kentucky, Lexington, KY.
Conditioning Medicine, 2019. 2(2):50-60.
Abstract
Regular aerobic exercise promotes anti-inflammatory effects, reduces stroke risk, and improves post-stroke outcomes. Rodent studies investigating exercise-induced neuroprotection, however, overlook clinically-relevant aspects of exercise training, including changes in intensity, volume, and detraining-related effects. This study used voluntary exercise training to determine how natural variations in exercise volume and detraining after exercise affect post-stroke neuroinflammation. Outbred Swiss Webster (SW) male mice (8-10 weeks old at start) were given unrestricted access to exercise wheels for 3 weeks. A second cohort of detrained mice exercised for 3 weeks and remained inactive for an additional 2 weeks. Sedentary control cohorts were housed for the same durations of 3 or 5 weeks. Flow cytometry evaluated adaptive and innate immune cells isolated from brain and spleen following a 60-min transient middle cerebral artery occlusion (tMCAo). High volume exercise reduced T cell populations in the uninjured brain, while exercise of a consistent volume was associated with activated splenic B cell populations. While exercise did not significantly reduce infarct volumes, detraining increased infarct volumes over both sedentary and exercise cohorts and elevated splenic CD4, CD8, and natural killer (NK) T cell populations at day 3 post-tMCAo. Detraining ablated exercise-induced leukocyte diapedesis into the ischemic hemisphere. Therefore, exercise training prior to stroke modulates both innate and adaptive post-stroke immune responses depending on variability in exercise volume and detaining. Understanding how variations in exercise affect neuroinflammation after stroke has significant clinical implications, given the high variation in adherence to exercise protocols for older adults at greater risk for stroke.
Keywords: Exercise training; stroke; neuroinflammation; detraining; preconditioning
Abstract
Regular aerobic exercise promotes anti-inflammatory effects, reduces stroke risk, and improves post-stroke outcomes. Rodent studies investigating exercise-induced neuroprotection, however, overlook clinically-relevant aspects of exercise training, including changes in intensity, volume, and detraining-related effects. This study used voluntary exercise training to determine how natural variations in exercise volume and detraining after exercise affect post-stroke neuroinflammation. Outbred Swiss Webster (SW) male mice (8-10 weeks old at start) were given unrestricted access to exercise wheels for 3 weeks. A second cohort of detrained mice exercised for 3 weeks and remained inactive for an additional 2 weeks. Sedentary control cohorts were housed for the same durations of 3 or 5 weeks. Flow cytometry evaluated adaptive and innate immune cells isolated from brain and spleen following a 60-min transient middle cerebral artery occlusion (tMCAo). High volume exercise reduced T cell populations in the uninjured brain, while exercise of a consistent volume was associated with activated splenic B cell populations. While exercise did not significantly reduce infarct volumes, detraining increased infarct volumes over both sedentary and exercise cohorts and elevated splenic CD4, CD8, and natural killer (NK) T cell populations at day 3 post-tMCAo. Detraining ablated exercise-induced leukocyte diapedesis into the ischemic hemisphere. Therefore, exercise training prior to stroke modulates both innate and adaptive post-stroke immune responses depending on variability in exercise volume and detaining. Understanding how variations in exercise affect neuroinflammation after stroke has significant clinical implications, given the high variation in adherence to exercise protocols for older adults at greater risk for stroke.
Keywords: Exercise training; stroke; neuroinflammation; detraining; preconditioning
Introduction
In the United States, stroke is the fifth leading cause of death and a leading cause of adult disability (Benjamin et al., 2017). Regular moderate intensity aerobic exercise is a well-established way to improve cardiovascular health and reduce the risk of stroke (Lee and Paffenbarger, 1998; Lee et al., 2003). In fact, individuals with active lifestyles prior to stroke have milder strokes, experience fewer motor deficits, and better functional recovery (Alevizos et al., 2005; Diep et al., 2010). Unfortunately, many individuals will begin, but fail to adhere to exercise regimens throughout their lives (Sherwood and Jeffrey, 2000). These attrition rates are particularly pronounced among older adults, a clinical population also at a higher risk for stroke (Chao et al., 2000). Two main approaches model exercise in animal studies: forced exercise (short bouts of treadmill running) and voluntary exercise (unrestricted access to running wheels). Studies using animal models of stroke confirmed that both forced and voluntary exercise are neuroprotective, reducing infarct volumes and promoting motor recovery, respectively (Egan et al., 2014). Though forced exercise protocols in rodents are effective neuroprotective strategies, voluntary exercise is more ‘physiologically relevant’, particularly with regard to the aforementioned variation in exercise volume, intensity, and duration in clinical populations. Few studies, however, harness these natural variations in animal models of voluntary exercise to better understand modulation of neuroprotection on post-stroke outcomes.
One of the benefits of exercise prior to stroke may be the global and multi-factorial anti-inflammatory effect of physical activity on the immune system (Gleeson et al., 2011). Exercise increases circulating innate and adaptive immune cell populations and alters leukocyte function in clinical populations (Sellami et al., 2018). This is particularly relevant for stroke recovery, as immediately after stroke there is a massive infiltration of peripheral immune cells into the ischemic brain (Schroeter et al., 1994). Initial inflammation is mediated predominantly by the innate immune system (e.g. neutrophils, monocytes, macrophages) (Del Zoppo et al., 2001), though lymphocytes from the adaptive immune system (e.g. B cells, T cells) secrete cytokines and produce reactive oxygen species (ROS) in an antigen-independent manner (Jin et al., 2010; Kim et al., 2014). These infiltrating immune cells activate resident brain immune cells (e.g. microglia) (Aktas et al., 2007) to release danger-associated molecular pattern molecules (DAMPs) and pro-inflammatory cytokines to exacerbate neuroinflammation (Liesz et al., 2015). Brain-derived antigens presented to T cells and B cells initiate the secondary adaptive immune response, specific to central nervous system (CNS) antigens (Ortega et al., 2015). In fact, three weeks of forced treadmill exercise in rats decreased general leukocyte infiltration into the brain after stroke (Ding et al., 2005), but a full survey of exercise-mediated changes in neuroinflammation is lacking.
Any anti-inflammatory, and potentially neuroprotective, effects of exercise are also dose-dependent (Gleeson et al., 2011). High intensity exercise training reduces circulating lymphocytes through increased apoptosis (Kruger and Mooren, 2014) and redistribution throughout lymphoid and non-lymphoid tissues (Kruger et al., 2008). Additionally, anti-inflammatory cytokines, such as interleukin (IL)-10 and transforming growth factor- β (TGF-β), increase after high intensity exercise, rendering individuals susceptible to illness (Gleeson et al., 2011; Wang et al., 2012; Gleeson et al., 2013; Perry et al., 2013; Kruger and Mooren, 2014). Given that high levels of IL-10 clinically increase the risk of post-stroke infection (Ashour et al., 2016), diminished immune function after high intensity exercise could actually exert a detrimental effect on post-stroke recovery. Indeed, some clinical studies suggest that moderate exercise may be more effective than high intensity exercise at reducing the risk of stroke (Lee and Paffenbarger, 1998; Deplanque et al., 2012). A period of inactivity after exercise, termed ‘detraining,’ can also counter exercise-mediated alterations to pro- and anti-inflammatory cytokines (Thompson et al., 1985; Rosety-Rodriguez et al., 2014; Steckling et al., 2016), suggesting that compliance to an exercise regimen is necessary for exercise-induced immunomodulation. Current animal models of exercise do not consider clinically-relevant aspects, such as exercise volume and detraining, when attempting to design mechanistic studies and therapeutic interventions. This may limit their translational relevance. We designed these studies to determine how natural variations in exercise volume and detraining, in a mouse model of voluntary exercise before stroke, mediate the post-stroke peripheral and neuroinflammatory responses for innate and adaptive immune populations. These results will aid in our understanding of endogenous changes in post-stroke neuroinflammation, and identify targets for future mechanistic studies of exercise-mediated neuroprotection.
Methods
Voluntary exercise training and detraining
Adult male Swiss Webster (SW) (Harlan Laboratories, Indianapolis, IN, USA) and C57Bl/6J (Jackson Labs, Bar Harbor, ME, USA) mice were randomly assigned to exercise, detraining, or control groups at 7-8 weeks of age (Supplemental Fig. 1A). During voluntary exercise training, mice were individually housed with unrestricted access to unlocked (freely moving) computer-monitored running wheels (Model #80820F, Camden Instruments Ltd., Loughborough, England) for 3 weeks. Because exercise was voluntary, mice were not coerced into a prescribed exercise regime and exercised ad libitum. An exclusion factor for voluntary wheel running averaging less than 10,000 wheel rotations/week was established a priori to exclude extremely inactive cases (2 mice removed from final analysis). Mice in the detraining cohort underwent the same duration of voluntary exercise training followed by a 2-week sedentary period with locked exercise wheels. Sedentary controls for both the exercise (3 weeks) and detraining (5 weeks) cohorts were individually housed for the same duration. Food and water were provided ad libitum and nestlets were provided to all groups for enrichment in cages with standard cob bedding. The Institutional Animal Care and Use Committee at the University of Texas Southwestern Medical Center (UTSW), Dallas, TX approved all procedures according to the Animal Welfare Act, PHS Animal Welfare Assurance requirements, and the ARRIVE guidelines. All mice were randomized, and all experimenters blinded to condition for data acquisition and analyses.

In a new window | Download PPT
Figure 1: Outbred mice exhibit higher exercise intensity. (A) Correlation plot for ‘exercise volume’, showing that average wheel rotations directly reflect exercise speed. (B) Average of 3 weeks of voluntary exercise for C57Bl/J6 (B6; circles) and Swiss Webster (SW; squares) mice. (C) Average exercise intensity for 3 weeks (3 grouped bars) for 3 representative B6 mice (white bars) and 3 representative SW mice (black bars). *p < 0.05. **p < 0.01 vs. B6 or as indicated by brackets.
Transient middle cerebral artery occlusion (tMCAo)
Mice were anesthetized (1.8-2% isoflurane/70% NO2/30% O2) while the body temperature was maintained at 37°C. Transcranial Laser Doppler flowmetry (Moor Instruments Ltd., Axminster, UK) established baseline middle cerebral artery (MCA) blood flow for each animal (Selvaraj et al. 2016). A 12 mm silicon coated 6.0 gauge nylon intraluminal filament (Washington University, St. Louis, MO, USA) was inserted into the common carotid artery to occlude the MCA for 60 minutes. Animals were placed in an incubator at 34°C for recovery during the occlusion period. The animals were re-anesthetized for the removal of the suture and reperfusion. Flowmetry was used to assess blood flow at the start and end of the occlusion period. Successful occlusion was defined as >80% reduction in MCA blood flow relative to the baseline for each animal. Reperfusion was defined as the return of blood flow to >50% of baseline. Mice not meeting the blood flow criteria were removed from the study at the time of surgery (16 out of 159 mice, 10%). Furthermore, 2 mice died during surgery, and 7 mice died after tMCAo.
Infarct Volume Quantification
Mice were sacrificed with isoflurane overdose 1 day after tMCAo. Brains were removed and sectioned into 1.0-mm thick coronal sections on a brain matrix. Sections were exposed to 2,2,3-triphenyl tetrazolium chloride (TTC; 2% TTC in 0.01M PBS; Sigma Aldrich, St. Louis, MO, USA), a compound that stains tissue with functional mitochondria red, while dead tissue remains white, allowing for the demarcation of infarct volumes. Infarct volumes were quantified (ImageJ) and corrected for edema based on the corresponding right hemispheric areas as control (Selvaraj et al. 2016).
Flow cytometry
Mice were sacrificed and perfused as previously described (Stowe et al., 2012). Brains were dissected and separated into ischemic and contralateral cortices. Brain tissue and spleens were manually dissociated and processed through a 70-μm mesh screen, washed in 0.01M PBS, and lymphocytes were isolated with a 30:70 discontinuous Percoll gradient by centrifugation (2,120g, 15 minutes, 21 oC, brake off; Eppendorf 5810R, Hamburg, Germany). After the lipid layer was removed, cells were resuspended in 1 mL of PBS and centrifuged (2120g, 15 minutes, 4oC, brakes off). Absolute cell counts in each sample were determined by Trypan Blue staining (20 μL Trypan Blue/180 μL sample) and counted. Spleens were then washed in 5 mL warmed Lympholyte (Cedar Lane) and centrifuged (1000g, 20 minutes, 21°C). Once the buffy layer was transferred to new lympholyte it was centrifuged (435g, 5 minutes, 21°C). Cellularity was determined and non-specific staining was blocked as previously described (Selvaraj et al. 2016). Both brains and spleens were stained using fluorescently labeled antibodies against CD45 (all leukocytes; A700), TCRβ (T cells; BV510), CD4 (CD4 T cells; v450), CD19 (B cells; PE), CD11b (innate immune cells; APC), GR-1 (granulated cells; FITC); NK1.1 (natural killer and natural killer T cells; PerCP-Cy5.5). After 45-minute incubation at 4oC, cells were washed with FACS wash buffer (PBS with 1% BSA) and fixed in 1% paraformaldehyde. All samples were run on a BD-FACS Aria flow cytometer (BD Biosciences, San Jose, CA, USA) using FACS Diva 6.0 software and data was analyzed using FlowJo (Tree Stars Inc., Ashland, OR, USA), with gating strategy shown in Supplemental Fig. 2.
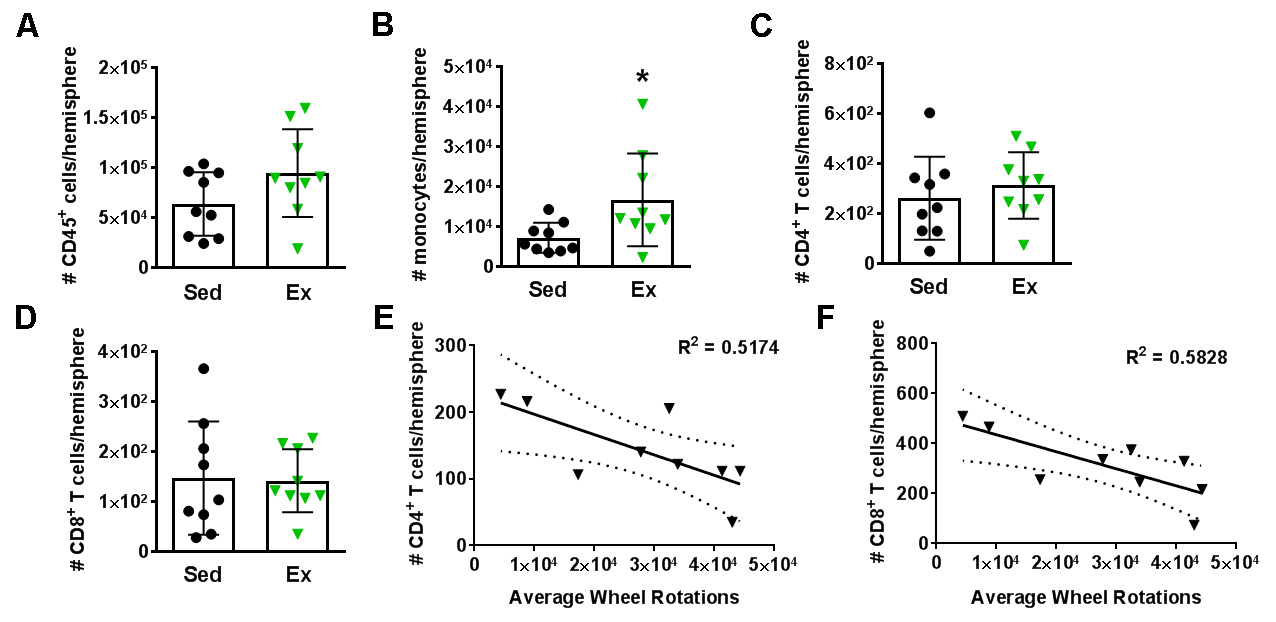
In a new window | Download PPT
Figure 2: High intensity voluntary exercise reduces T cells in the brain. Hemispheric immune cell populations for sedentary (Sed; black circles; n = 9) and exercise (Ex; green triangles; n = 9) cohorts after 3 weeks of voluntary exercise show increases in (A) CD45+ general leukocytes and (B) monocytes, but not (C) CD4 T cells and (D) CD8 T cells. When the Ex data are plotted according to average wheel rotations over 3 weeks, both (E) CD4 and (F) CD8 T cells are reduced in the brains of animals that ran at a higher intensity.*p < 0.05.
Microarray analysis
Splenic CD19+ B cells were isolated one day after wheel lock following 3 weeks of voluntary exercise. Control B cells were isolated from sedentary controls. RNA was isolated from each sample and underwent two rounds of amplification prior to quantification on an Illumina MouseWG-2 V2 array (San Diego, CA, USA) at the UTSW Microarray Core. Detectable genes were normalized (Partek Genomics Suite, St. Louis, MO, USA), with final between-group significance of genes detected determined via a restricted maximum likelihood (REML) one-way analysis of variance (ANOVA). Gene ontology pathways were analyzed using Ingenuity Pathway Analysis (IPA, Qiagen, Valencia, CA, USA), with a priori choice of genes p < 0.0001 and associated with a canonical pathway in the IPA Knowledge Base used for software analysis.
Statistics
Differences in infarct volume, splenic immune cell populations, and brain immune cell populations were compared using a one-way ANOVA (Graphpad Prism; La Jolla, CA, USA). The relationship between wheel rotations and leukocyte populations in the brain, and the relationship between wheel rotations and leukocyte populations in the spleen, were analyzed using linear regressions. Post-stroke leukocyte egress displayed a non-linear correlation that was analyzed using a second order univariate polynomial nonlinear regression. Inter-hemispheric neuroinflammation was evaluated with a paired t-test. Outliers were removed from analysis based on the ROUT analysis in Prism, which included 5 mice based on infarct volume and 5 mice based on general CD45+ leukocyte numbers from the post-stroke brain. Significance was considered as p < 0.05, whereas a trend was considered p < 0.06.
Results
Outbred SW mice exercise more than C57Bl/6J mice
Because exercise volume may be a critical mediator of exercise-induced immunomodulation, we first established that the average number of wheel rotations over 1 week is highly correlated to the averaged running speed (R2 = 0.997; Fig. 1A). In this study, to quantify both exercise intensity and duration, we used the measurement of “average wheel rotations/week” to represent “exercise volume”. Voluntary and forced exercise performance also differs between mouse strains, including strain differences in running duration, distance, and average speed (Lerman et al., 2002). In our model, both SW and C57Bl/6J (B6) mice were given ad libitum access to running wheels for 3 weeks. SW mice ran at a greater exercise volume (p < 0.01; Fig. 1B) and with significantly greater variations in exercise, including a 2.5 fold increase from the lowest to the highest-volume runners (Fig. 1C). Given our hypothesis that exercise volume will modulate the immune system, SW mice were chosen as the more appropriate strain for our study.
High volume voluntary exercise alone alters adaptive cellularity in the brain
In order to determine the effect of exercise training on innate and adaptive immunity after stroke, it was necessary to establish if exercise training alone alters the immune system in the absence of injury. Our flow cytometry panel identified innate cell subsets, including neutrophils, monocytes, macrophages, and NK cells, as well as adaptive immune cell subsets, including B cells, IL-10+ regulatory B cells (Bregs), CD4 (i.e. helper) T cells, CD8 (i.e. cytotoxic) T cells, and NK T cells. Three days after completion of 3 weeks of voluntary exercise (Ex), we examined adaptive and innate immune cells in the brain and spleen. There were no changes in splenic immune cell populations in Ex mice compared to sedentary (Sed) controls (population data, Supplementary Fig. 3). However, within the same mice, monocytes were increased in the brain (p < 0.05) in Ex mice relative to Sed mice (Fig. 2A). No other changes were observed in leukocyte populations in the brain (Supplementary Fig 3). While exercise did not exert an effect on any splenic populations, high volume exercise was inversely correlated with CD4 (R2 = 0.58; p < 0.05) and CD8 T cells (R2 = 0.52; p < 0.05) populations in the brain (Fig. 2 C-F). As exercise volume increased, CD4 and CD8 T cells were diminished, indicating a CNS-specific change in the adaptive immune system - in the absence of injury - mediated by exercise volume and not identified by population analyses. This dose-dependent response did not occur for any other immune cell subset in either the brain or spleen (Supplemental Table 1).
Consistent exercise alters B cell-related signaling
We originally hypothesized that B cell gene expression may be affected by exercise, as we previously identified unique B cell phenotypes following another form of preconditioning that induces neuroprotection from stroke (Monson et al., 2014). Therefore, after exercise training, microarray analysis was performed on splenic B cells from Ex and Sed mice (Fig. 3). Given that exercise volume modulated adaptive immunity, we hypothesized that Ex animals would exhibit a unique B cell phenotype as a group. However, we found that it was not exercise itself, but instead consistent exercise over 3 weeks that mediated changes in B cell gene expression. Ex mice that decreased exercise volume over the course of 3 weeks were phenotypically closer to Sed mice instead of mice that maintained a constant level of exercise, regardless of intensity (Fig. 3A, B). Comparison of gene expression showed that for similarly clustered Ex (animals #3-5) and Sed (animals # 1,3,4) mice, exercise altered expression of 3,175 out of 18,099 genes, including a greater than 2-fold upregulation of 181 genes, and greater than 2-fold downregulation of 108 genes. The top 10 up- and down-regulated genes identified are shown in Fig. 3D.
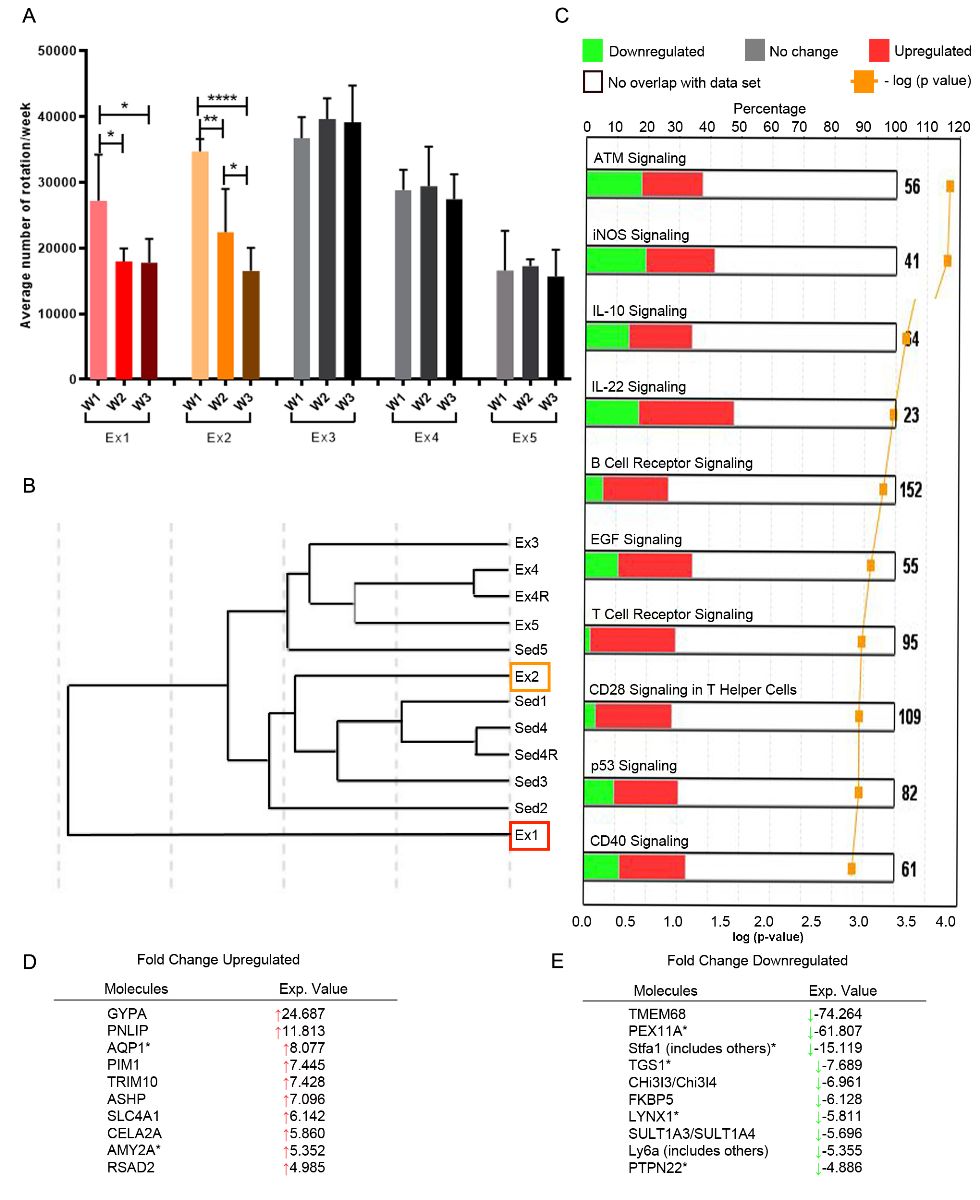
In a new window | Download PPT
Figure 3: B cell gene expression is altered by consistent exercise. (A) Mice (n=5) ran at variable intensities over the course of three weeks (W; bars show mean +/- standard deviation for 7 days wheel running). Splenic exercise (Ex) B cells were isolated and analyzed with microarray to compare to sedentary (Sed) expression (n = 5). (B) B cells from animals with decreasing exercise intensity (red, orange boxes correspond to red, orange bar graphs) were phenotypically closer to sedentary (Sed) mice. (C) Upregulation (red) and downregulation (green) of B cell-related canonical pathways, plus the top 10 (D) upregulated and (E) downregulated genes (fold-change over sedentary) are shown. * p < 0.05; ** p < 0.01; **** p < 0.0001.
Notably, consistent exercise activated several canonical B cell pathways, including B cell receptor (BCR) and CD40 (a stimulatory protein found on antigen presenting cells) signaling, in addition to activation of IL-10 and IL-22 cytokine signaling, the latter cytokine associated with protection following hepatic ischemia (Fig. 3C) (Eggenhofer et al., 2016). Upregulation of BCR and CD40 signaling may enhance interactions between B cells and T cells, but when combined with the 7-fold increase in PIM1 expression, a gene that encodes a serine-threonine kinase in myeloid cells, also promotes the survival and proliferation of B cells (Zhu et al., 2002). There was a 4-fold decrease in expression of the protein tyrosine phosphatase nonreceptor type 22 (PTPTN22) gene, linked to a risk for autoimmune diseases (Arechiga et al., 2009; Menard et al., 2011). PTPTN22 regulates T cell and B cell tolerance by decreasing T cell receptor (TCR) and BCR signaling and diminishing B cell proliferation (Menard et al., 2011). In contrast, genes associated with the IL-10 signaling pathways were both upregulated and downregulated, making the total outcome of this alteration in signaling unclear. However, the predicted IL-10 signaling pathway increases IL-10rα and IL-10rβ, which would inhibit the synthesis of pro-inflammatory cytokines and promote B cell activation (Supplementary Fig. 4) (Moore et al., 2001). In summary, a consistent volume of exercise over the entirety of exercise training is associated with an activation of several anti-inflammatory gene programs in peripheral CD19+ B cells, but these effects are lost as exercise volume wanes over time, and do not alter the number of B cells recruited into the brain.
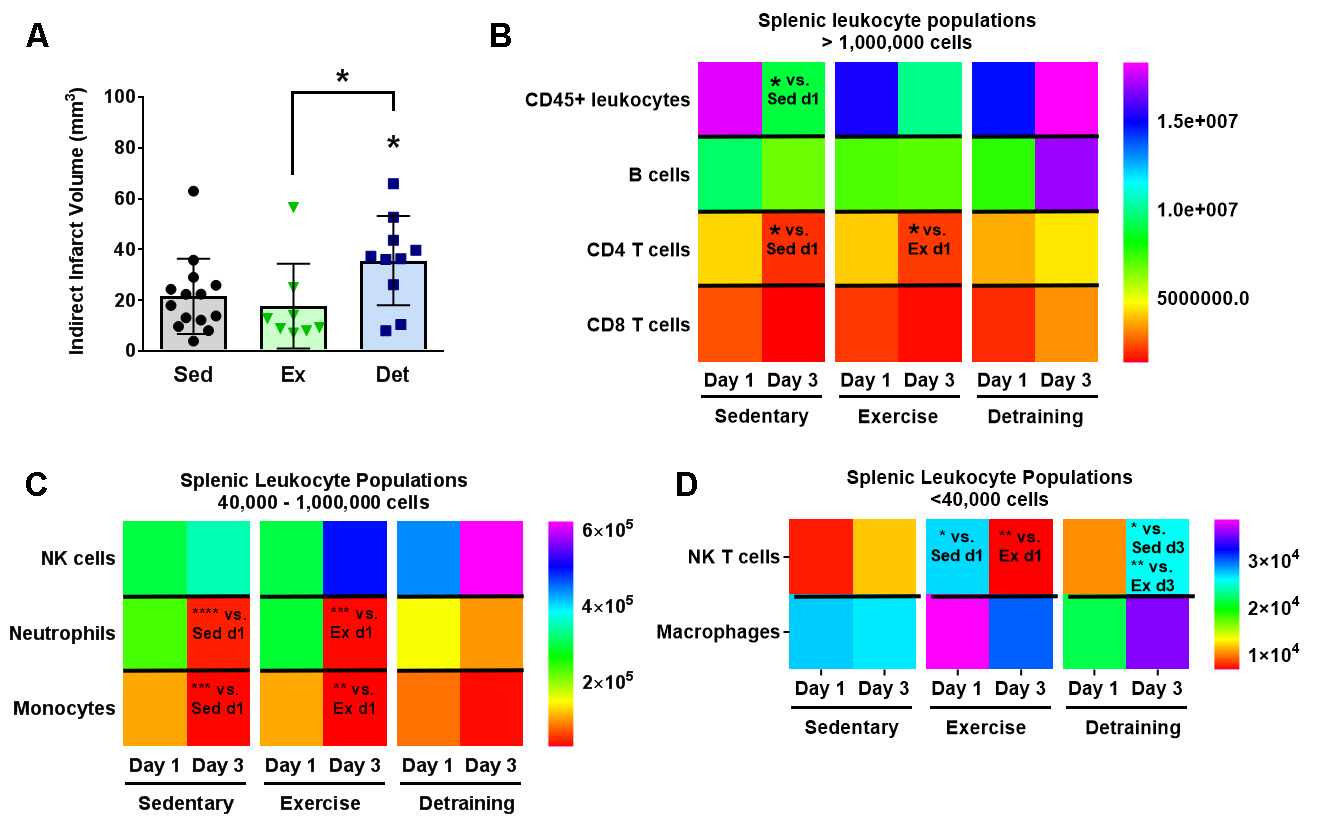
In a new window | Download PPT
Figure 4: Detraining after voluntary exercise increases infarct volumes and splenic T cells. (A) Detraining in mice (blue squares, n = 10) increases infarct volumes (mean ± standard deviation) compared to sedentary (black circles, n = 14) and exercise (green triangles, n = 8) cohorts. * p < 0.05 vs. sedentary cohort, or as indicated by bracket (B-D) Heat maps for splenic populations, with corresponding individual population data shown in Supplemental Fig. 5. In general, splenic populations are significantly reduced at day 3 post-stroke compared to day 1. Detrained animals do not exhibit this reduction in splenic numbers, and actually exhibit higher CD4, CD8, and NK T cell populations on day 3 after stroke. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 versus (vs.) group as indicated by text.
Detraining exacerbates infarct volume and splenic NK T cell populations
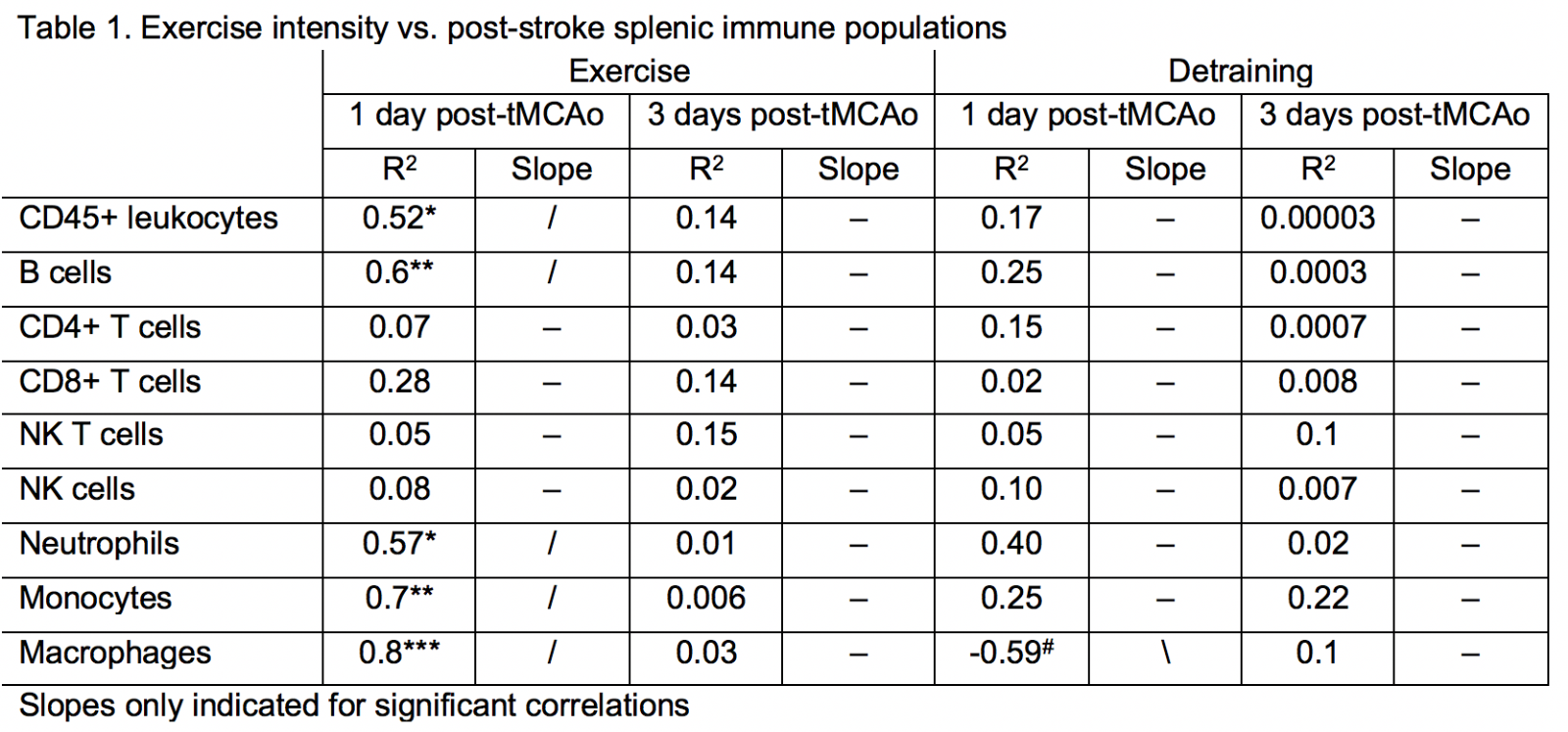
Given that decreasing exercise volume actively counters exercise-mediated gene expression, we implemented a detraining (Det) protocol of 3 weeks of voluntary exercise training followed by a 2-week sedentary period to determine the longevity of exercise-mediated neuroprotection (schematic, Supplemental Fig. 1A). This is particularly relevant given adherence failure to exercise regimens for many people, including those at high risk of stroke (Chao et al., 2000; Sherwood and Jeffrey, 2000). Voluntary exercise for 3 weeks prior to stroke onset did not reduce infarct volumes one day post-stroke (17 mm3 ± 15 mm3; Fig. 4A) compared to Sed controls (20 mm3 ± 14 mm3; p = 0.69). However, detraining doubled infarct volumes (36 mm3 ± 18 mm3) over both Ex (p < 0.05) and Sed (p < 0.05) cohorts, suggesting that detraining after voluntary exercise exacerbates stroke injury. There was, however, no relationship between stroke size and exercise volume in either Ex (p = -0.22) or Det (p = 0.035; Supplementary Fig. 5).
Detraining also impacted post-stroke changes in splenic leukocyte populations at 1 and 3 days post-stroke. Splenic population heat maps are shown in Fig. 4 and general population analysis shown in Supplemental Fig. 6. We found a general decrease in both Sed and Ex cohorts comparing post-stroke day 3 to day 1 for CD45+ leukocytes (Sed: p < 0.05; Ex p = 0.06), CD4+ T cells (both p < 0.05), neutrophils (Sed: p < 0.0001; Ex: p < 0.001) and monocytes (Sed: p < 0.001; Ex: p < 0.01). After detraining, this peripheral immunosuppression is not only lost, but NK T cell populations were enhanced in Det mice 3 days after tMCAo compared to both Sed mice (p < 0.05) and Ex mice (p < 0.01; Supplementary Fig. 6E). Det mice also showed a trend towards increases in CD4 and CD8 T cells (both p = 0.06) in the spleen compared to Sed mice.
If the same data are correlated to exercise volume, mice with a higher exercise volume had a greater number of multiple innate immune cell subsets, including neutrophils (R2 = 0.57), monocytes (R2 = 0.7), and macrophages (R2 = 0.8), retained in the spleen at day 1 following stroke, but not at day 3 post-stroke (Table 1). Of the adaptive immune cells, only B cells correlated with exercise volume at 1 day post-stroke (R2 = 0.6). Similar to innate immune cells, lower exercise volume was associated with fewer B cells in the spleen. The immediate effects of exercise volume on post-stroke peripheral inflammation were lost after detraining, with the exception of macrophages. Interestingly, detraining induced the opposite effect, as there was a trending decrease in macrophages in Det mice that ran more (R2 = 0.59).
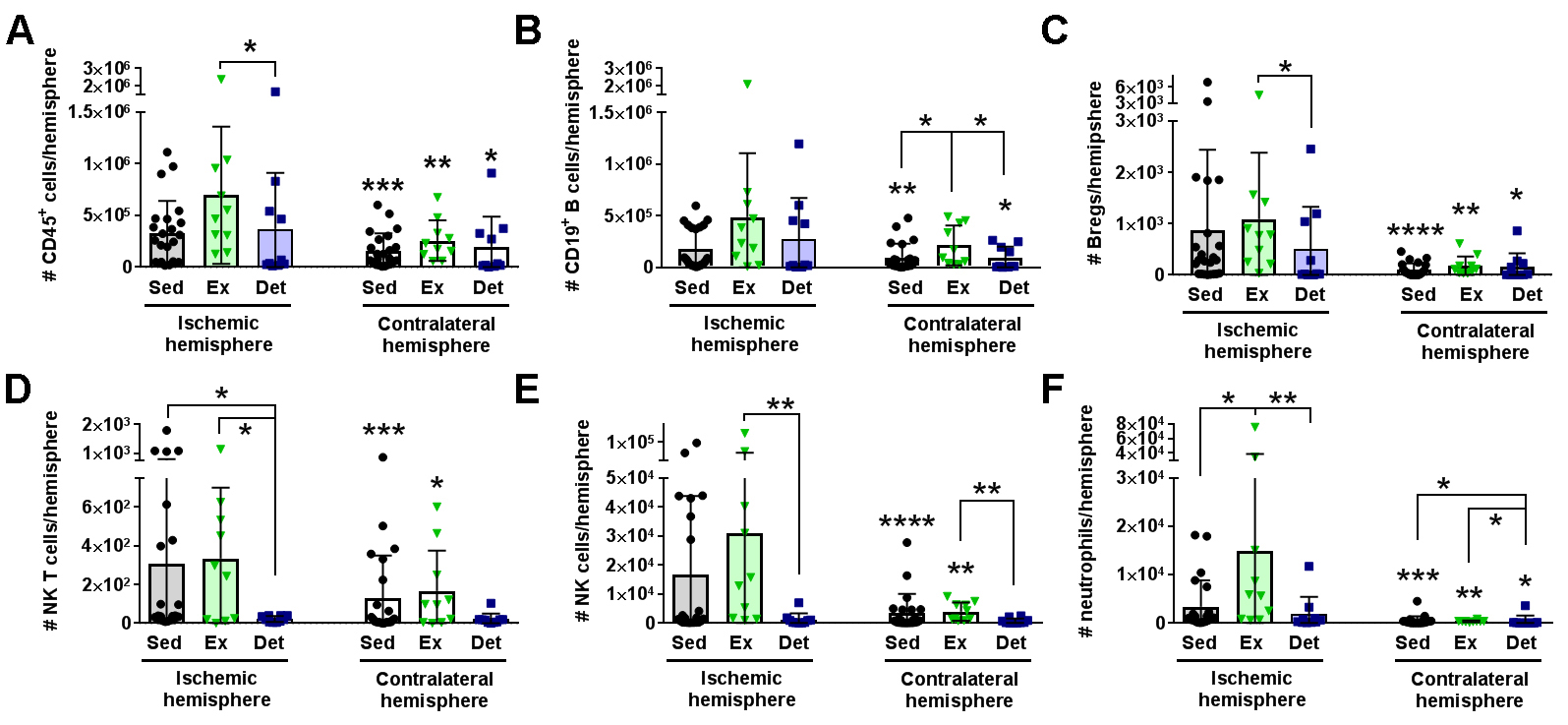
In a new window | Download PPT
Figure 5: Detraining suppresses leukocyte diapedesis into the brain 3 days after stroke. Data graphed for sedentary (Sed; black circles; n = 23), exercise (Ex; green triangles; n = 9) detraining (Det; blue squares; n = 13) populations in the ischemic hemisphere (left bars) and the uninjured contralateral hemisphere (right bars). In general, there was significant leukocyte egress in the ischemic hemisphere compared to the contralateral hemisphere. Exercise increased the specific diapedesis of (A) CD45+ general leukocytes, (E) NK cells, and (F) neutrophils into the ischemic brain over both the Sed or Det cohorts. Det mice exhibited reduced egress of (C) B regulatory (Breg) cells and (D) NK T cells. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. ischemic hemisphere for brain unless otherwise indicated by a bar.
Detraining suppresses leukocyte diapedesis into the ischemic brain.
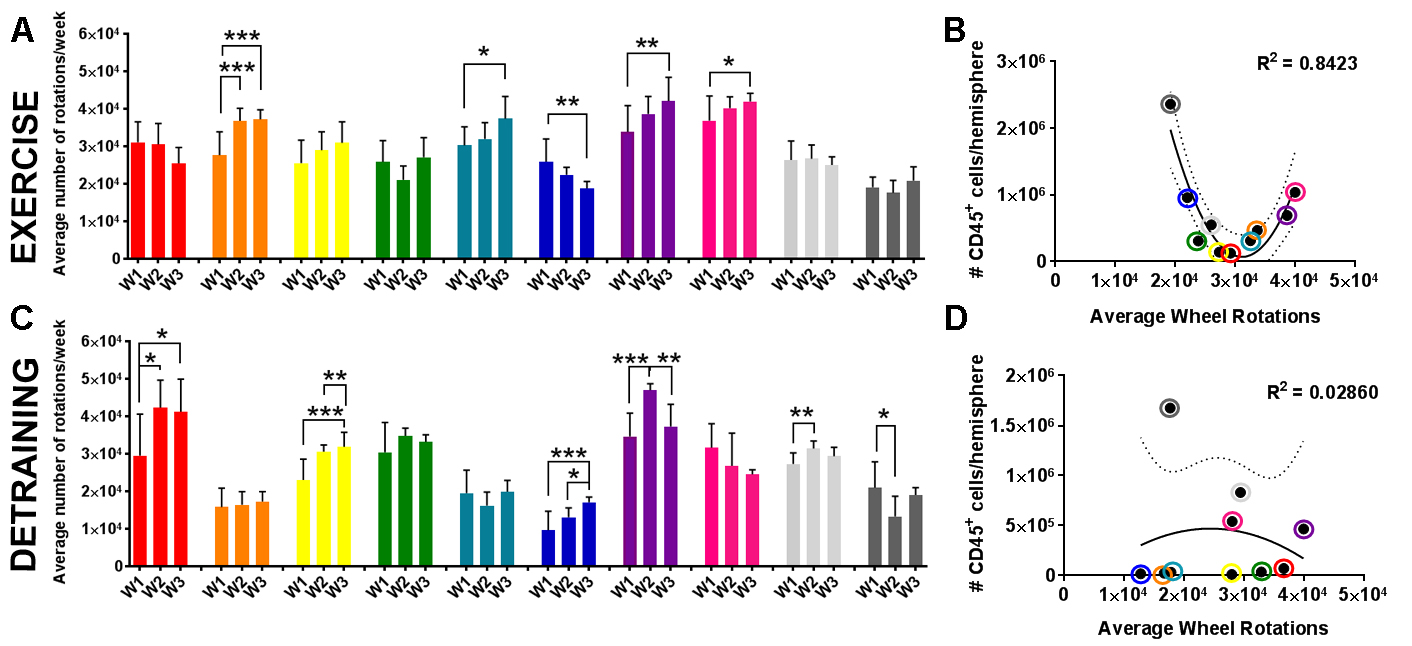
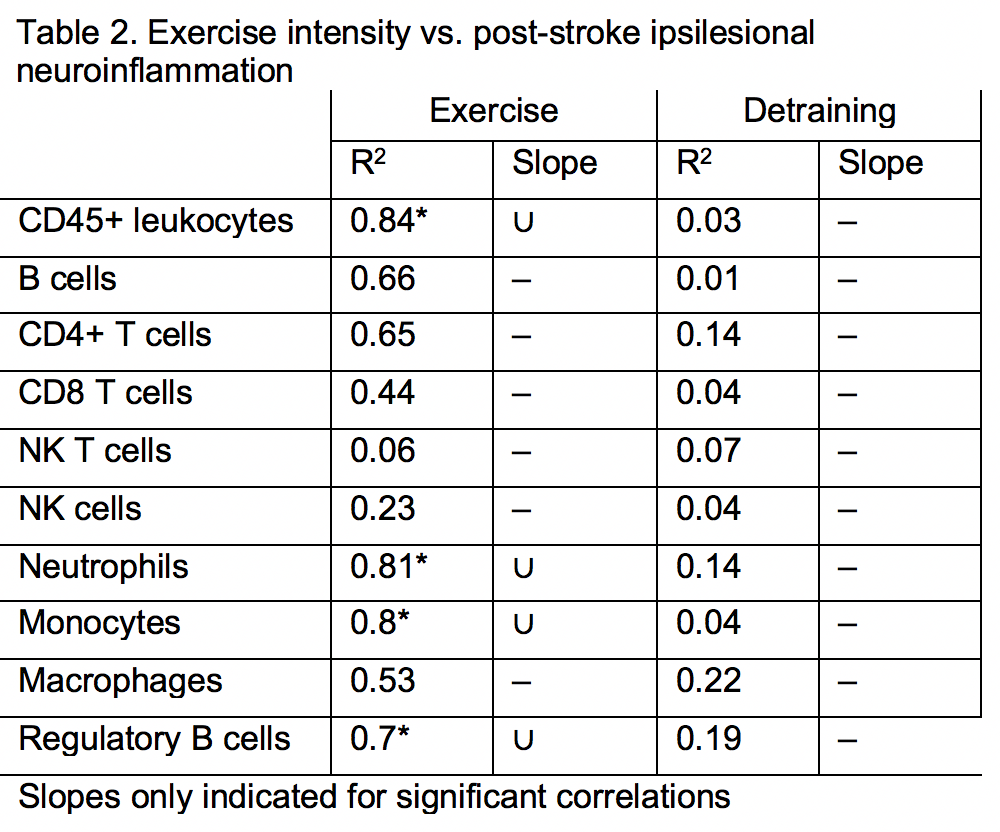
Finally, we examined the effect of exercise volume and subsequent detraining on post-stroke leukocyte diapedesis. As expected, most leukocyte subsets exhibited significantly higher infiltration into the ischemic (i.e. injured) hemisphere over the contralateral (i.e. uninjured) hemisphere (Fig. 5). Exercise training increased the diapedesis of neutrophils (p < 0.05 vs. Sed; p < 0.01 vs. Ex) and NK cells (p = 0.06 vs. Sed; p < 0.01 vs. Det) into the ipsilesional hemisphere (Fig. 5D-F). Of all adaptive immune cell subsets, detraining reduced infiltration into the ipsilesional hemisphere for Bregs (p < 0.05 vs. Ex; Fig. 5C) and NK T cells (p < 0.05 vs. both Sed and Ex; Fig. 5D). Ipsilesional egress of T cell populations, monocytes, and macrophages were unaffected by exercise training or detraining (Supplemental Fig. 7). Exercise volume mediated recruitment of immune cell populations into the ischemic hemisphere 3 days after tMCAo. Exercise volume induced a non-linear, dose-dependent effect (Fig. 6). Mice that exercised at either a high or low intensity had a greater number of CD45+ leukocytes (R2 = 0.84; Fig. 6B; Table 2), in the brain, while these populations were suppressed in moderately exercising animals. As previously observed, detraining ablated all effects of exercise volume on the immune system as exercise volume no longer correlated with changes in leukocytes (R2 = 0.0.03; Fig. 6D).
In a new window | Download PPT
Figure 6: Detraining ablates the effect of exercise volume on leukocytes in the brain. (A) Exercise (Ex; n = 10) and (C) detraining (Det; n = 10) animals ran at variable intensities (bars show mean ± standard deviation for 7 days wheel running) for 3 weeks (W). (B) There was non-linear relationship between CD45+ leukocyte egress into the brain and exercise intensity, that (D) was lost after 2 weeks of detraining. Individual animals identified by colored circles corresponding to the same colored bars of wheel data in (A, C).
Discussion
Noncompliance to exercise regimens is a significant problem among clinical populations, particularly for hypertensive and elderly patients who are at a greater risk for stroke (Ahmed et al., 2008). Our study is the first to examine the effect of exercise volume and detraining on ischemic injury after stroke, with an emphasis on modulation of the immune system during voluntary exercise. Surprisingly, we found that detraining resulted in significantly larger infarct volumes compared to both exercise and sedentary conditions, with a concomitant loss of almost all exercise-induced changes in post-stroke neuroinflammation. It should be noted that our model of voluntary exercise did not significantly decrease infarct volumes compared to sedentary controls, similar to other studies demonstrating differential effects of forced and voluntary exercise - both before and after stroke - on neuroprotection (Ke et al., 2011; Egan et al., 2014). While animals that undergo forced exercise training have greater reductions in infarct volume, voluntary exercise improves neurobehavioral scores and motor recovery, often in the absence of significant effects on infarct volume. Forced exercise is a stressful paradigm, however, that increases corticosterone levels, which impair B cell activation, proliferation and differentiation, and worsens cognitive deficits after stroke (Sugo et al., 2002; Ke et al., 2011; Egan et al., 2014; Becker et al., 2016). This highlights the novelty of our finding that animals with only 2 weeks of detraining following 3 weeks of voluntary exercise exhibit such deleterious effects on infarct volumes, and suggests an active and detrimental response to stroke activated after the cessation of exercise.
At first glance, voluntary exercise only modestly affects post-stroke inflammation, similar to the aforementioned effects on infarct volume. In fact, grouped analyses demonstrate that exercise only increased neutrophil populations in the ischemic hemisphere over sedentary and detrained cohorts. However, when considering a much more subtle mechanism - exercise volume - voluntary exercise training significantly modulates many other post-stroke adaptive and innate immune cell populations. In the spleen, higher volume exercise correlated with increased B cell and innate immune populations, due to either proliferation or retention. In the brain, exercise volume exerted a non-linear, dose-dependent effect on immune cells infiltrating into the ischemic hemisphere. Low and high volume exercise increased these immune cells in the post-stroke brain, while moderate volume exercise suppressed their recruitment. Thus, exercise volume determined which subset of immune cells was modulated (T cells vs. innate immune cells and B cells), which organs were affected (CNS vs. CNS and spleen), and the nature of the relationship between volume and immunity (linear relationships in the spleen vs. non-linear relationships in the CNS). These factors must be considered in designing voluntary exercise studies in mice, and may have contributed to the mixed results in previous voluntary exercise studies (Austin et al., 2014).
In our study, 3 weeks of voluntary wheel running results in differential modulation of CD4 T cell and Breg populations. In the absence of injury, there are no changes to either CD4 T cell populations in the brain or spleen of exercised animals when analyzed as a group, consistent with other research in rats following forced treadmill running (Shabkhiz et al., 2008). Unlike Bregs, however, high exercise volume correlated with changes in CD4 T cell recruitment to the brain, but not spleen, indicating a CNS-specific phenomenon, as fewer CD4 T cells were present in high-volume runners. Future studies should determine which subsets of CD4 T cells are modulated by exercise volume and recruited into the brain, especially as elevations of CD4 T cells after stroke can be either detrimental or beneficial, depending on the subset, location, and timing of diapedesis (Kleinschnitz et al., 2010; Stubbe et al., 2013; Brea et al., 2014; Romer et al., 2015). Bregs are also beneficial to recovery after stroke (Ren et al., 2011; Bodhankar et al., 2013; 2014), and were significantly and selectively increased in the ischemic hemisphere of exercised animals compared to detrained mice. In rats that underwent high intensity treadmill running, there was also an increase in regulatory T cells (Shabkhiz et al., 2008), suggesting that exercise may increase several subsets of IL-10-producing adaptive immune cells (Bodhankar et al., 2015). Our microarray data suggest several mechanisms by which this may happen, including activation of BCR and CD40 signaling. Bregs require BCR signaling and CD40 stimulation for development and suppressive functions (Mauri and Bosma, 2012). CD40 stimulation of B cells by T cells induces a cascade of events resulting in B cell activation, protection against apoptosis, and maturation into antibody producing cells (Kehry, 1996). Exercise-induced activation of pathways like CD40 suggests that T cells may be an important immune population after exercise and could contribute to exercise-induced neuroprotection, while alterations in B cells may exhibit a concomitant immunosuppressive phenotype to minimize further injury. Future studies should further confirm our microarray findings using different methodologies, including qPCR analysis of B cell and T cell populations before and after exercise.
While studies consistently show that high intensity/high volume exercise training suppresses immune function in elite athletes and increases the likelihood of infection (Gleeson et al., 2011), very little research has investigated the mechanisms responsible for the dose-dependent effect of exercise volume on neuroinflammation. One potential explanation for dose-dependent effects may be exercise-induced changes in chemokines, a class of cytokines known to recruit immune cells to the site of ischemic injury after stroke (Chang et al., 2015; Nieman et al., 2015; Ross et al., 2016; Barry et al. 2017). CCL2, a chemokine that promotes the diapedesis of innate immune cells and T cells into the brain (Stowe et al., 2012; Cedile et al., 2017), is a particularly promising candidate because it is necessary for preconditioning-induced neuroprotection against stroke (Stowe et al., 2012), and is modulated by exercise in a dose-dependent manner (Peake et al., 2005). High intensity exercise increases CCL2 protein levels in plasma and skeletal muscle in endurance-trained athletes (Peake et al., 2005; Nieman et al., 2015; Wells et al., 2016). In contrast, moderate exercise has the inverse effect on the CCL2/CCR2 axis. Moderate training reduces plasma levels of CCL2 and decreases surface expression of CCR2 on monocytes (Many et al., 2013). Given that higher serum levels of CCL2 are associated with poorer post-stroke outcome and recurrence, suppression of CCL2 by moderate exercise training could be beneficial (Chen et al., 2018). Together, these data demonstrate that exercise volume differentially alters CCL2 levels in serum and remote tissues, as well as induces phenotypic and functional changes to leukocytes. Future studies should determine if other chemokines are similarly moderated by exercise volume which, when coupled with stroke-induced chemokine expression, could exert significant impact on post-stroke neuroinflammation.
In addition to exercise volume and intensity, one must consider how detraining affects the immune system. Our study is the first to examine detraining-induced modulation of neuroinflammation in mice, finding that two weeks of detraining abolished several key alterations to the immune system by exercise training. First, detraining caused retention of splenic immune cells 3 days after stroke. Given the loss of high volume exercise-induced diapedesis from the spleen to the ischemic hemisphere, there were concomitantly fewer immune cells in the brain of Det mice compared to Ex mice. Elevation of splenic NK cells and NK T cells by detraining could contribute to T cell-mediated immunosuppression, as seen with several immune populations in the ischemic hemisphere of Det mice. Studies in rats following distal focal stroke demonstrate that NK cells and T cells contribute to immunosuppression independent of effects on infarct volume (Gu et al., 2013), and human studies confirm that invariant NK T cells are linked to stroke severity and severe immunosuppression in patients (Wong et al., 2017). Thus, investigation into the long-term consequences of detraining on adaptive immunity and recovery after stroke is needed, including future studies to identify exercise-mediated mechanisms that are disrupted in uninjured animals.
Summary
The 4th leading risk factor for global death, according to the World Heatlth Organization, is physical inactivity (Benjamin et al., 2017). A sedentary lifestyle significantly increases the likelihood of stroke, and studies indicate that the benefits of exercise are dose-dependent (Benjamin et al., 2017). However, it is currently unclear what the ideal intensity, duration, frequency, and importantly, volume, of exercise training is necessary to confer the benefits of exercise. Additionally, adherence to exercise regimens are a large problem in the clinic (Chao et al., 2000). Therefore, it is critical to understand how natural variations in voluntary exercise affect post-stroke outcome. We found that in mice that underwent voluntary exercise training, there was a dose-dependent and volume-mediated change in immune cell recruitment from the spleen to the ischemic brain. Furthermore, detraining worsened stroke outcome, potentially by the ablation of neuroprotective immunomodulation by pre-stroke exercise training. Further research is needed to understand exercise-induced alterations to immunity in an effort to maximize the neuroprotective effects of exercise training.
Acknowledgments
Thank you to the UT Southwestern Neuromodels facility for their assistance in housing the mice and stroke surgeries. Additional thanks to Grant Jenkins, Sara Ireland, PhD, Ding Chen, PhD, Ibrahim Noorbhai, MD, Neha N. Mithani, Alex Partin, Felonie Doss, and Angelica McPartlin, MD for their assistance in processing samples, running experiments, and care of the mice. We would also like to acknowledge the contributions of the UTSW Microarray core for processing and analysis of our microarray samples.
Grant Funding
Funding has been generously provided by the American Physiological Society (KP), American Heart Association 14SDG18410020 (AMS), and the Beatrice Menne Haggerty Endowment for Stroke Research.
Conflicts of interest
The authors have no competing interests with publication of this manuscript.
References
Katherine Poinsatte1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Sterling B. Ortega1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Uma M. Selvaraj1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Anouk J. M. Meeuwissen1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Xiangmei Kong1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Erik J. Plautz1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Nancy L. Monson1
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
Rong Zhang1,2
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
2Institute for Exercise and Environmental Medicine, Texas Health Presbyterian Hospital, Dallas, TX, USA.
Ann M. Stowe1,3
1Department of Neurology & Neurotherapeutics, UT Southwestern Medical Center, Dallas, TX, USA.
3Department of Neurology, University of Kentucky, Lexington, KY.
Corresponding author:
Ann Stowe
Email: ann.stowe@uky.edu

In a new window | Download PPT
Figure 1: Outbred mice exhibit higher exercise intensity. (A) Correlation plot for ‘exercise volume’, showing that average wheel rotations directly reflect exercise speed. (B) Average of 3 weeks of voluntary exercise for C57Bl/J6 (B6; circles) and Swiss Webster (SW; squares) mice. (C) Average exercise intensity for 3 weeks (3 grouped bars) for 3 representative B6 mice (white bars) and 3 representative SW mice (black bars). *p < 0.05. **p < 0.01 vs. B6 or as indicated by brackets.

In a new window | Download PPT
Figure 2: High intensity voluntary exercise reduces T cells in the brain. Hemispheric immune cell populations for sedentary (Sed; black circles; n = 9) and exercise (Ex; green triangles; n = 9) cohorts after 3 weeks of voluntary exercise show increases in (A) CD45+ general leukocytes and (B) monocytes, but not (C) CD4 T cells and (D) CD8 T cells. When the Ex data are plotted according to average wheel rotations over 3 weeks, both (E) CD4 and (F) CD8 T cells are reduced in the brains of animals that ran at a higher intensity.*p < 0.05.

In a new window | Download PPT
Figure 3: B cell gene expression is altered by consistent exercise. (A) Mice (n=5) ran at variable intensities over the course of three weeks (W; bars show mean +/- standard deviation for 7 days wheel running). Splenic exercise (Ex) B cells were isolated and analyzed with microarray to compare to sedentary (Sed) expression (n = 5). (B) B cells from animals with decreasing exercise intensity (red, orange boxes correspond to red, orange bar graphs) were phenotypically closer to sedentary (Sed) mice. (C) Upregulation (red) and downregulation (green) of B cell-related canonical pathways, plus the top 10 (D) upregulated and (E) downregulated genes (fold-change over sedentary) are shown. * p < 0.05; ** p < 0.01; **** p < 0.0001.

In a new window | Download PPT
Figure 4: Detraining after voluntary exercise increases infarct volumes and splenic T cells. (A) Detraining in mice (blue squares, n = 10) increases infarct volumes (mean ± standard deviation) compared to sedentary (black circles, n = 14) and exercise (green triangles, n = 8) cohorts. * p < 0.05 vs. sedentary cohort, or as indicated by bracket (B-D) Heat maps for splenic populations, with corresponding individual population data shown in Supplemental Fig. 5. In general, splenic populations are significantly reduced at day 3 post-stroke compared to day 1. Detrained animals do not exhibit this reduction in splenic numbers, and actually exhibit higher CD4, CD8, and NK T cell populations on day 3 after stroke. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 versus (vs.) group as indicated by text.

In a new window | Download PPT
Figure 5: Detraining suppresses leukocyte diapedesis into the brain 3 days after stroke. Data graphed for sedentary (Sed; black circles; n = 23), exercise (Ex; green triangles; n = 9) detraining (Det; blue squares; n = 13) populations in the ischemic hemisphere (left bars) and the uninjured contralateral hemisphere (right bars). In general, there was significant leukocyte egress in the ischemic hemisphere compared to the contralateral hemisphere. Exercise increased the specific diapedesis of (A) CD45+ general leukocytes, (E) NK cells, and (F) neutrophils into the ischemic brain over both the Sed or Det cohorts. Det mice exhibited reduced egress of (C) B regulatory (Breg) cells and (D) NK T cells. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001 vs. ischemic hemisphere for brain unless otherwise indicated by a bar.

In a new window | Download PPT
Figure 6: Detraining ablates the effect of exercise volume on leukocytes in the brain. (A) Exercise (Ex; n = 10) and (C) detraining (Det; n = 10) animals ran at variable intensities (bars show mean ± standard deviation for 7 days wheel running) for 3 weeks (W). (B) There was non-linear relationship between CD45+ leukocyte egress into the brain and exercise intensity, that (D) was lost after 2 weeks of detraining. Individual animals identified by colored circles corresponding to the same colored bars of wheel data in (A, C).


Supporting Information
Download Supporting Information (PDF)Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 9301 | 19 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA