Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Repeated hypoxic preconditioning dynamically changes synaptic vesicle distribution in the mouse olfactory bulb
Time:2020-01-07
Number:7465
Yi Liu1, Qian Li2, Zhishan Sun3, Jianwen Lin1, Jun Zhao4, Jiandong Yu5, Changhong Ren6, Xunming Ji7
Author Affiliations


- 1Department of Neurology, Dalian Municipal Central Hospital, Affiliated Hospital of Dalian Medical University, Dalian 116033, China.
- 2Neuroscience Research Institute, Peking University, Beijing, China.
- 3Department of Neurosurgery, Weifang People's Hospital, Weifang 261000, PR China.
- 4Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, 10029 USA.
- 5Guangdong-Hongkong-Macau Institute of CNS Regeneration, Jinan University, Guangzhou, China.
- 6Beijing Key Laboratory of Hypoxia Conditioning Translational Medicine, Beijing, China.
- 7Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Conditioning Medicine 2019. 2(6): 280-283.
Abstract
Repeated hypoxic preconditioning (HPC) induces neuroprotection against subsequent ischemic/hypoxic events. This neuroprotective effect can be observed as fast as one hour after HPC treatment in mice. Synaptic vesicles (SVs), which change dynamically minutes to hours after HPC, might serve as the mechanism for HPC-induced neuroprotection. However, this potential mechanism has not been previously examined. Therefore, in this study we used a conventional repeated HPC mice model to investigate SVs number and distribution by transmission electron microscopy (TEM), as well as the protein levels of synapse related markers, including soluble N-ethylmaleimide sensitive fusion attachment protein receptors (SNARE) and synaptophysin, in synaptosomal fractions and whole cell lysates of the olfactory bulb (OB) by western blots.
The results from transmission electron microscopic and western blot analyses confirmed the SVs number, and the SV markers, synaptophysin and vesicle associated membrane protein-2 (VAMP2), decreased significantly after 1-3 cycle of HPC, but returned to baseline levels after 5-cycle HPC treatment, while other synapse related markers were unaffected. Further TEM observation confirmed that SVs in the reserve pool, rather than in the readily releasable pool or recycling pool, accounted for the change in the total SVs during repeated HPC treatment. Dynamic changes in SVs distribution during repeated HPC indicated enhanced synaptic plasticity, which could serve as the underlying mechanism of HPC-induced neuroprotection.
Keywords: Hypoxic preconditioning, synaptic vesicle distribution, reserve pool
Abstract
Repeated hypoxic preconditioning (HPC) induces neuroprotection against subsequent ischemic/hypoxic events. This neuroprotective effect can be observed as fast as one hour after HPC treatment in mice. Synaptic vesicles (SVs), which change dynamically minutes to hours after HPC, might serve as the mechanism for HPC-induced neuroprotection. However, this potential mechanism has not been previously examined. Therefore, in this study we used a conventional repeated HPC mice model to investigate SVs number and distribution by transmission electron microscopy (TEM), as well as the protein levels of synapse related markers, including soluble N-ethylmaleimide sensitive fusion attachment protein receptors (SNARE) and synaptophysin, in synaptosomal fractions and whole cell lysates of the olfactory bulb (OB) by western blots.
The results from transmission electron microscopic and western blot analyses confirmed the SVs number, and the SV markers, synaptophysin and vesicle associated membrane protein-2 (VAMP2), decreased significantly after 1-3 cycle of HPC, but returned to baseline levels after 5-cycle HPC treatment, while other synapse related markers were unaffected. Further TEM observation confirmed that SVs in the reserve pool, rather than in the readily releasable pool or recycling pool, accounted for the change in the total SVs during repeated HPC treatment. Dynamic changes in SVs distribution during repeated HPC indicated enhanced synaptic plasticity, which could serve as the underlying mechanism of HPC-induced neuroprotection.
Keywords: Hypoxic preconditioning, synaptic vesicle distribution, reserve pool
Introduction
Ischemic/hypoxic preconditioning (I/HPC) is defined as a sublethal exposure that elicits tissue or organ resistance to subsequent more drastic ischemic or hypoxic insults (Murry et al., 1986). A bilateral preconditioning device to prevent recurrent stroke in intracranial arterial stenosis has been developed and clinically validated in patients (Meng et al., 2012).
HPC exerts neuroprotective effects against subsequent ischemic stroke 24 hours later in rodents (Gidday et al., 1994), while repeated HPC triggers neuroprotection as fast as 1 hr after treatment (Zhang et al., 2011). The latter finding provoked more studies to investigate the mechanisms that underlie the rapid neuroprotective effects of HPC. Our previous studies identified concomitant changes in synaptic curvature and mitochondrial distribution during repeated HPC (Liu et al., 2015), suggesting alterations in synaptic responses to HPC. Synaptic vesicles (SVs) carry various chemical messages between neurons, which are highly susceptible to ischemia (Kawakami et al., 2001; Khatri and Man, 2013). Synaptic plasticity plays pivotal roles in maintaining normal brain function under physiological and pathological conditions. However, up to now, little is known about the dynamic changes in SVs during HPC.
The primary aim of this study was to elucidate how SV number and distribution change during repeated HPC, which could potentially provide a mechanism for its neuroprotection.
Materials and methods
Animals
Male ICR mice weighing 21 - 26 g were obtained from Beijing Vitalriver Experimental Animal Co. (Beijing, China). Animals were randomly group-housed in a standard animal care room with temperature maintained at 22 ± 1°C, humidity 50 ± 5%, and 12-hour light/dark cycle. Animals were divided into four groups: control, 1-cycle, 3-cycle, and 5-cycle HPC treatment groups. Each group contained at least 12 mice: 3 mice were for transmission electron microscopy (TEM) analysis, and 9 other mice were for western blot analysis of whole cell lysates (n = 3) and synaptosomal fraction (n = 6) from the olfactory bulb (OB). The mice were acclimatized to the environment and were provided with chow and water ad libitum except when they were subjected to HPC treatment. All procedures in this study were conducted according to the guidelines set by the University Animal Care and Use Committee of Capital Medical University and were consistent with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Hypoxic preconditioning treatment
Experiments were conducted at room temperature (18-22°C) on adult male ICR mice weighing 18-22 g. HPC treatment was performed as described previously [5]. For the 1-cycle HPC treatment, mice were placed individually in a 125 ml bottle with fresh air and sealed with screw cap. Once mice began gasping, tolerance time was recorded and mice were removed from the sealed bottle and allowed to recover for 30 minutes under normoxic conditions. For 3- and 5-cycle HPC treatments, this procedure was repeated for 3 and 5 times, respectively. New bottles were used between cycles. Right after the last 30-min recovery time for each group, mice were sacrificed with an intraperitoneal injection of chloral hydrate (350 mg/kg) and decapitated.
Synaptosome preparation and western blot analysis
OB whole cell lysate and synaptosomal fractions were prepared separately, and western blots were performed as previously described (Liu et al., 2009; 2010). The glial marker, glial fibrillary acidic protein (GFAP), and synaptic vesicle marker, synaptophysin, were used to confirm successful isolation of synaptosomes.
TEM sample preparation and analysis
OB samples for TEM analysis were prepared as previously described (Liu et al., 2015). Only Type I synapses were included for SVs analyses in this study. Seventeen to twenty TEM pictures were analyzed for each group. Total SV number and their distances to the active zone were carefully measured. Further analyses compared SV number in the readily releasable pool (RRP), recycling pool, and the reserve pool. RRP and recycling pool were defined as those SVs attached to presynaptic membrane or within 300 nm from the active zone respectively, and all the remaining SVs were referred to as the reserve pool (Sudhof, 2004). SV analysis and quantification were analyzed by professional technicians blind to the experimental conditions.
Data Analysis
All data were expressed as group mean ± standard error of the mean (SEM). Data from western blots and TEM analyses were evaluated by unpaired T-test. Statistical significant difference was set at p < 0.05.
Results
As more cycles of HPC treatment were performed (Fig. 1), tolerance time increased significantly (Liu et al., 2015). TEM analysis revealed that SV number in OB decreased significantly after 3-cycle HPC and then return to normal levels after 5-cycle HPC treatment, while the active zone length was not affected (Fig. 2A & B). SV number was evaluated by TEM and western blot analyses. The synaptosomal fraction was confirmed with the glial marker, GFAP, and synaptic vesicle marker, synaptophysin. Results show enrichment of synaptophysin and a decrease in GFAP in the synaptosomal fraction, which indicate successful isolation of synaptosomes in our study (Fig. 2C). Western blot analysis of OB synaptosomes showed that the SV markers, synaptophysin and vesicle-associated membrane protein 2 (VAMP2), first decreased and then returned to normal levels during repeated HPC treatment (Fig. 2D & E), which matched the pattern observed with TEM. Syntaxin1, Synaptosomal-associated protein 25 (SNAP25), and Munc18, synaptic markers that reside on presynaptic membranes, were unaffected in the synaptosomal fraction (Fig. 2D & E). Protein levels of synapse related proteins, Syntaxin1, Munc18, and VAMP2 from whole OB lysate were also analyzed by western blots and were found to be unaffected during repeated HPC treatment (Fig. 3).
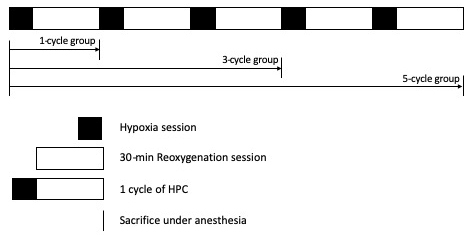
In a new window | Download PPT
Figure 1: Hypoxic preconditioning (HPC) protocol. Filled and blank rectangular boxes refer to hypoxia sessions and 30-min reoxygenation sessions respectively, which together comprise a 1-cycle HPC. Mice were sacrificed at the end of the reoxygenation session in each group.
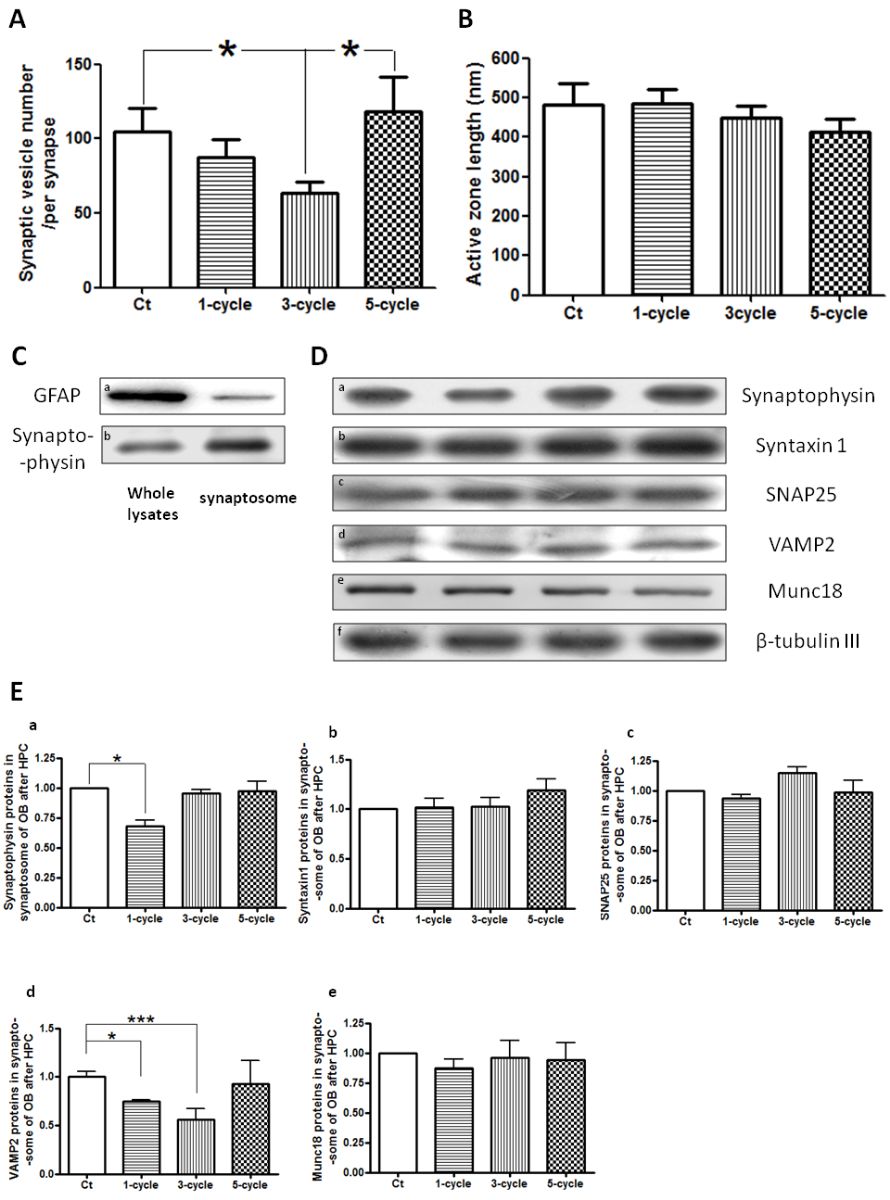
In a new window | Download PPT
Figure 2: SV number changes during repeated HPC. TEM analyses showed that SV number decreased significantly after 3-cycle HPC treatment and then returned to normal levels after 5-cycle HPC treatment (A). At the same time, the active zone length was unaffected (B). The synaptosomal fraction was confirmed with enriched synaptophysin and less GFAP compared to whole cell lysate (C). Western blot analysis detected synapse related proteins in synaptosome (D). Statistical analyses showed the SV markers in the synaptosome, synaptophysin and VAMP2, decreased and then returned to normal levels during repeated HPC, while other synapse related markers, Syntaxin1, SNAP25, and Munc18, which reside on presynaptic membrane, were unaffected (E).
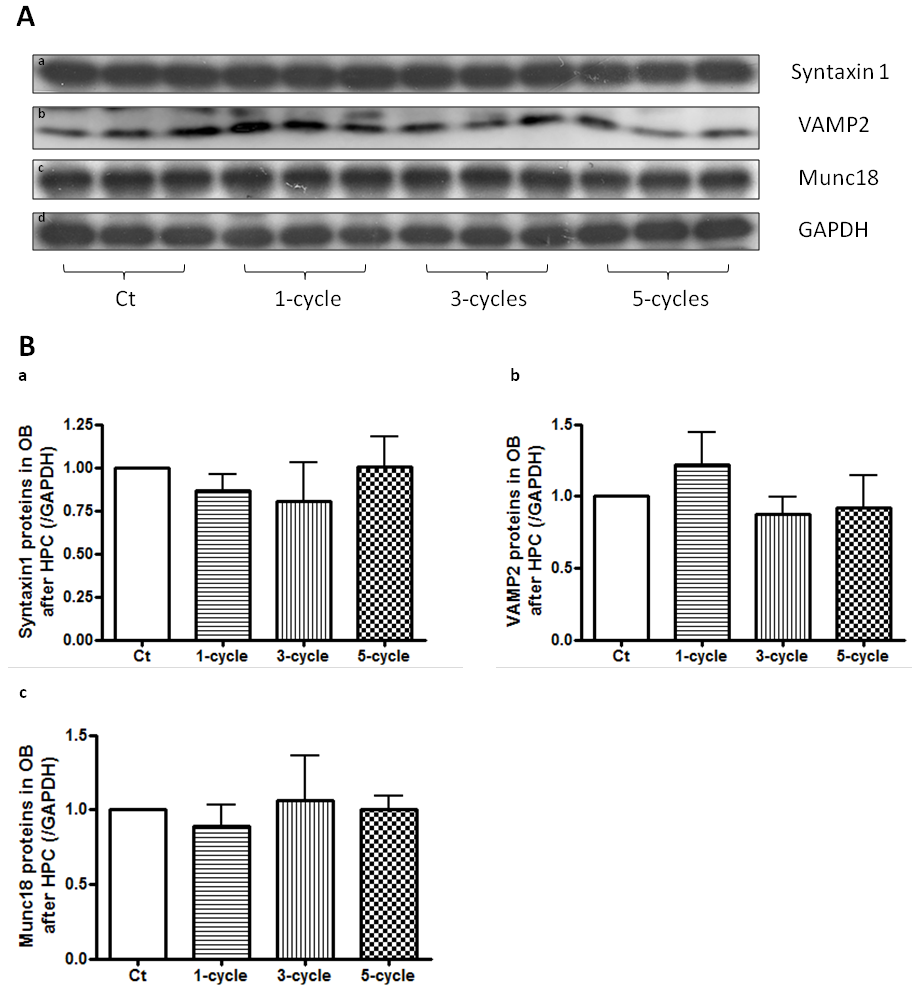
In a new window | Download PPT
Figure 3: Synapse related proteins in OB whole cell lysates are unaffected during repeated HPC. Western blot analysis detected synapse related protein markers from OB whole cell lysate (A). Statistical analyses revealed that they were unaffected during repeated HPC (B).
TEM was further used to analyze SV distribution (i.e. number and distances to the active zone). Changes observed in SVs in the reserve pool matched the changes observed in the total SV pool, which decreased significantly after 3-cycle HPC treatment and returned to normal levels after 5-cycle HPC treatment. Neither SVs in RRP nor in the recycling pool were significantly changed during repeated HPC treatments (Fig. 4).
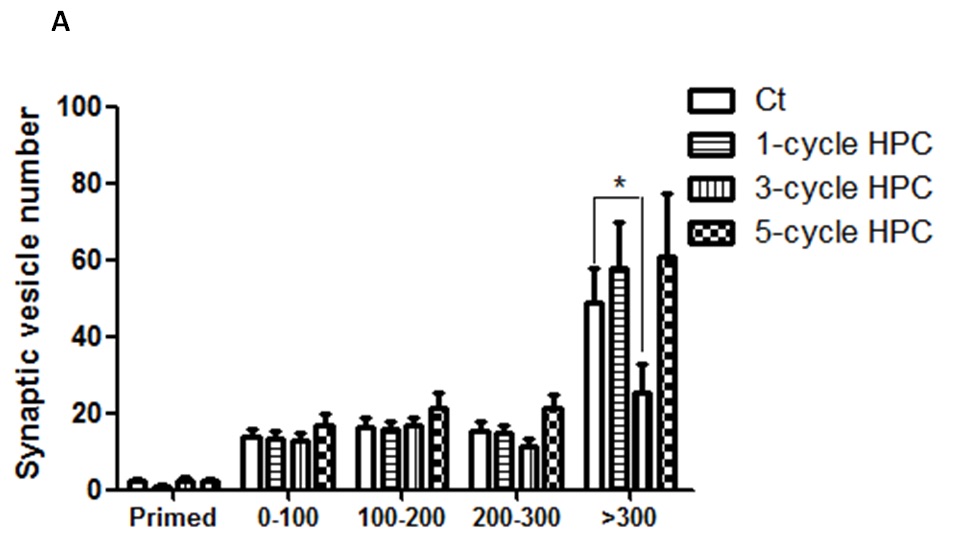
In a new window | Download PPT
Figure 4: Changes in SVs distribution during repeated HPC treatment.
TEM was used to observe and record SVs distribution. Statistical analyses showed that SVs mainly the in reserve pool were affected by HPC exposure, rather than in readily releasable SV pool or the recycling pool.
Discussion
In this study, we identified a decrease and then restoration of SV number during repeated HPC, with the change mainly occurring in the SV reserve pool. HPC triggered neuroprotective effects following subsequent ischemic stroke, which occurred within 1 hour and 24 hours later (Gidday et al., 1994; Zhang et al., 2011), which correlated with underlying time-dependent neuroprotective mechanisms involving immediate release of mediators and activation of signaling pathways for the early phase (1 hr), and de-novo protein synthesis for the delayed phase (24 hr) (Tsai et al., 2004). Repeated HPC, performed within 200 minutes, improved long-term potentiation and cognitive ability (Shao et al., 2006). Relevant mechanistic studies in to synaptic plasticity are rare. Our previous study identified dynamic changes in synaptic curvature during repeated HPC concurrent with alterations in the percentage of synapses with presynaptic mitochondrial changes, while the synaptic number remained unaffected (Liu et al., 2015). Although synaptic number correlates closely with cognition, it takes time to have cytoskeleton re-organization. In this case, more presynaptic vesicle mobilization could be responsible for long-term potentiation (LTP) and cognitive improvement. The current study revealed dynamic changes in synaptic vesicle number, mainly located in the reserve pool.
Presynaptic vesicles are distributed in three functionally distinct pools, including the RRP (5-8 vesicles), which is comprised of active zone SVs fully primed for immediate release, the recycling pool (20-60% SVs) that undergo continuous exocytotic/endocytotic cycling, and the reserve pool (40-80% SVs) that is reluctant to mobilization unless subjected to prolonged stimulation (Rizzoli and Betz, 2005; Alabi and Tsien, 2012).
SVs in the RRP were considered to be the vesicles that participate in instantaneous synaptic activity and maintain physiological brain function. Recruitment of SVs from the reserve pool into the recycling pool is central to synaptic plasticity. Synaptic transmission is highly energy consuming, and presynaptic release is more vulnerable than the postsynaptic response to ischemic conditions (Bolay et al., 2002). A deficiency in the mitochondria in synapses resulted in the inability to mobilize the reserve pool after intense stimulation, while basic SV activity was unaffected (Verstreken et al., 2005). This study confirmed a decrease and then an increase in SVs in the reserve pool during repeated HPC accompanies the increase and then a decrease in presynaptic mitochondria number (Liu et al., 2015), which provides a mechanism for this energy consuming procedure.
SV fusion is driven by assembly of the SNARE complex, a four-helical bundle composed of syntaxin 1 and SNAP25 on the plasma membrane, and VAMP2 on the vesicle membrane (Verhage and Toonen, 2007). Synaptosome, an isolated synaptic terminal, represents local alterations in synaptic activity better than whole cell preparations. This study confirmed VAMP2 and synaptophysin, located on presynaptic vesicle, decreased in synaptosomes but were unaffected in whole cell lysates, indicating a decrease in SV number instead of protein levels caused by repeated HPC, which coincided with electron microscopic observation of synaptic vesicle number per synapse. An increase in the number of SVs doesn’t necessarily mean greater neuroprotective effects, however, it does facilitate synaptic plasticity under conditions of damage or severe stimulation. How SVs are mobilized is still controversial (Chanaday et al., 2019). Currently it’s technically not feasible to observe SV mobilization simultaneously with HPC treatment. However, understanding how SV mobilization occurs under HPC treatment is a focus of our ongoing work.
Acknowledgment
This work was supported by Liao Ning Revitalization Talents Program (grant number XLYC 1807083); National Natural Science Foundation of China (grant number 81200915), National Natural Science Foundation of China (grant number 81671288), and Dalian Medical Sciences Research Project (grant number 1811010).
Competing Interests
The authors declare they have no competing financial interest.
References
Yi Liu1
1Department of Neurology, Dalian Municipal Central Hospital, Affiliated Hospital of Dalian Medical University, Dalian 116033, China.
Qian Li2
2Neuroscience Research Institute, Peking University, Beijing, China.
Zhishan Sun3
3Department of Neurosurgery, Weifang People's Hospital, Weifang 261000, PR China.
Jianwen Lin1
1Department of Neurology, Dalian Municipal Central Hospital, Affiliated Hospital of Dalian Medical University, Dalian 116033, China.
Jun Zhao4
4Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, 10029 USA.
Jiandong Yu5
5Guangdong-Hongkong-Macau Institute of CNS Regeneration, Jinan University, Guangzhou, China.
Changhong Ren6
6Beijing Key Laboratory of Hypoxia Conditioning Translational Medicine, Beijing, China.
Xunming Ji7
7Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, China.
Corresponding author:
Xunming Ji
Email: jixm@ccmu.edu.cn
or
Yi Liu
Email: letaliu@bjmu.edu.cn

In a new window | Download PPT
Figure 1: Hypoxic preconditioning (HPC) protocol. Filled and blank rectangular boxes refer to hypoxia sessions and 30-min reoxygenation sessions respectively, which together comprise a 1-cycle HPC. Mice were sacrificed at the end of the reoxygenation session in each group.

In a new window | Download PPT
Figure 2: SV number changes during repeated HPC. TEM analyses showed that SV number decreased significantly after 3-cycle HPC treatment and then returned to normal levels after 5-cycle HPC treatment (A). At the same time, the active zone length was unaffected (B). The synaptosomal fraction was confirmed with enriched synaptophysin and less GFAP compared to whole cell lysate (C). Western blot analysis detected synapse related proteins in synaptosome (D). Statistical analyses showed the SV markers in the synaptosome, synaptophysin and VAMP2, decreased and then returned to normal levels during repeated HPC, while other synapse related markers, Syntaxin1, SNAP25, and Munc18, which reside on presynaptic membrane, were unaffected (E).

In a new window | Download PPT
Figure 3: Synapse related proteins in OB whole cell lysates are unaffected during repeated HPC. Western blot analysis detected synapse related protein markers from OB whole cell lysate (A). Statistical analyses revealed that they were unaffected during repeated HPC (B).

In a new window | Download PPT
Figure 4: Changes in SVs distribution during repeated HPC treatment.
TEM was used to observe and record SVs distribution. Statistical analyses showed that SVs mainly the in reserve pool were affected by HPC exposure, rather than in readily releasable SV pool or the recycling pool.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 7465 | 20 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA