Browse Articles
Conditioning Medicine
International bi-monthly journal of cell signaling, tissue protection, and translational research.
Current Location:Home / Browse Articles
Reconditioning fatty livers: closing the gap between supply and demand in transplantation
Time:2020-07-03
Number:8910
Christoph Emontzpohl1, Steve Bynon2, Christian Stoppe3, Gernot Marx3, Cynthia Ju1
Author Affiliations


- 1Department of Anesthesiology, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
- 2Department of Surgery, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
- 3Department of Intensive and Intermediate Care, University Hospital Aachen, Aachen, Germany.
Conditioning Medicine 2020. 3(3):135-143.
Abstract
Nonalcoholic fatty liver disease (NAFLD) is progressively growing worldwide, being one of the main reasons for liver transplantation. New strategies are needed to overcome this problematic development and some studies are showing promising results. Apart from that, high mortality on the liver transplant list due to prevalent organ shortage, has led to the consideration of using marginal grafts (e.g. steatotic livers) for transplantation. However, fatty livers are more susceptible to injuries related to ischemia/reperfusion, resulting in higher rates of posttransplant complications.
Growing evidence suggests that reduced tolerance of steatotic livers to ischemia could potentially be mitigated after successful preservation and processing. Different strategies, like machine perfusion and the usage of de-fattening cocktails, are being considered to overcome these problems.
This review highlights potential agents for future treatment strategies of nonalcoholic steatohepatitis (NASH) and focuses on the clinical application of machine perfusion to increase the number of acceptable organs for transplantation and improve overall patient outcome.
Keywords: liver transplantation, fatty liver, machine perfusion, reconditioning, steatosis
Abstract
Nonalcoholic fatty liver disease (NAFLD) is progressively growing worldwide, being one of the main reasons for liver transplantation. New strategies are needed to overcome this problematic development and some studies are showing promising results. Apart from that, high mortality on the liver transplant list due to prevalent organ shortage, has led to the consideration of using marginal grafts (e.g. steatotic livers) for transplantation. However, fatty livers are more susceptible to injuries related to ischemia/reperfusion, resulting in higher rates of posttransplant complications.
Growing evidence suggests that reduced tolerance of steatotic livers to ischemia could potentially be mitigated after successful preservation and processing. Different strategies, like machine perfusion and the usage of de-fattening cocktails, are being considered to overcome these problems.
This review highlights potential agents for future treatment strategies of nonalcoholic steatohepatitis (NASH) and focuses on the clinical application of machine perfusion to increase the number of acceptable organs for transplantation and improve overall patient outcome.
Keywords: liver transplantation, fatty liver, machine perfusion, reconditioning, steatosis
Introduction
Orthotopic liver transplantation (OLT) is the only treatment for patients with end-stage liver disease. In 2018, 8,250 livers were transplanted and 11,844 new waitlist registrants were added to the list in the USA. Although short- and long-term survival improved during the last decades, limited organ availability still leads to a waiting list mortality of about 20% (Northup et al., 2015; Trapero-Marugan et al., 2018). The increasing gap between supply and demand of organs leads to strategies like living donation, splitting of grafts for two recipients, or the transplantation of marginal organs in extended criteria donor (ECD) programs. In many cases, characteristics include advanced donor age, extended duration of cold storage (>12 hours), macrosteatosis of more than 30%, or mixed steatosis of more than 60% (Tector et al., 2006; Durand et al., 2008). Due to an increasing number of steatotic livers/patients, it will be especially important to overcome challenges on this topic.
To this day, static cold storage (SCS) is commonly used in transplantation (Belzer and Southard, 1988; Ploeg et al., 1992). Through hypothermia, SCS decreases the metabolism and oxygen demand to increase the potential ischemic time without rapid impairment of the graft. However, metabolism is not completely stopped, due to anaerobic metabolism leading to a depletion of adenosine triphosphate (ATP). Moreover, the disrupted blood flow leads to a lack of shear stress and impaired endothelial cells and nitric oxide (NO) synthesis (Upadhya et al., 2003; Peralta et al., 2013). As ECD allografts are more susceptible to ischemia/reperfusion injury (IRI), the current clinical strategies of SCS cannot fulfil the demands presented by their transplantation. Improvements in organ preservation are therefore an increasing focus in research. This review aims to present some of the current strategies in reconditioning fatty livers, with a special focus on clinical aspects and machine perfusion.
Pathophysiology in fatty liver disease
Several factors have been reported to be associated with steatotic liver disease. These factors include oxidative stress, lipotoxicity, molecular factors, or the distribution pattern of accumulated fat.
1. Macro- vs. microvesicular steatosis
Macrovesicular steatosis is the most common form of fatty liver related injuries and is caused by accumulating intracellular lipids due to an oversupply in the liver, leading to disturbed lipid metabolism. The accumulation of lipids leads to increasing hepatocyte volume. The subsequent blockage of the sinusoid space increases the vascular resistance of the microcirculation, eventually leading to a detrimentally impaired supply of oxygen and nutrients, especially when considering less tolerance of the steatotic liver during IRI (Fukumori et al., 1999; Seifalian et al., 1999; Ijaz et al., 2003; Farrell et al., 2008). While macrovesicular steatosis seems to be potentially reversible, microvesicular steatosis is caused by the accumulation of liposomes in the cytoplasmic compartments of the cells, which may result from impaired mitochondria β-oxidation of fatty acids (Reddy and Rao, 2006).
Although today it is thought that liver grafts with mild steatosis (<30% macrosteatosis) and microsteatosis do not lead to an increased risk of primary graft nonfunction (PNF) or early allograft dysfunction (EAD), there is still some controversy (Briceño et al., 2005; Spitzer et al., 2010; Dutkowski et al., 2012; Andert et al., 2017). Croome et al. (2019) found that after receiving microsteatotic livers, patients showed an increased rate of postreperfusion syndrome (PRS), EAD, and continuous renal replacement therapy (CRRT) following liver transplantation, compared to patients receiving non-steatotic livers. Nonetheless, there was no difference in patient or graft survival (Croome et al., 2019). Conversely, moderate steatosis (>30% macrosteatosis) was shown to be an independent risk factor for EAD (Spitzer et al., 2010; Dutkowski et al., 2012). Moreover, a transplant with moderate macrosteatosis is more likely to lead to post-transplant complications, such as cardiac arrest or a postreperfusion syndrome, compared to transplants with mild or no steatosis (Linares et al., 2019).
2. Lipotoxicity and oxidative stress
Lipotoxicity refers to cellular injury/death caused by accumulation of free fatty acids or their metabolites. The underlying reasons for lipotoxicity are still not completely clarified (Hauck and Bernlohr, 2016). It is thought to induce hepatocellular death, thereby activating resident Kupffer cells and causing an inflammatory response (Trauner et al., 2010). Although this is debated, an accumulation of triglycerides due to a disturbed ability to utilize, store, or export them, might at least lead to compression of the sinusoids and changes in blood flow (Bass, 2010). However, mice with increased triglyceride formation/accumulation alone did not show increases in the pro-inflammatory pathways of c-Jun N-terminal kinase (JNK) phosphorylation or nuclear factor-κB (NF-κB) activation, both markers that are typically elevated in steatohepatitis, suggesting JNK activation specifically in NASH, but not NAFLD (Monetti et al., 2007; Puri et al., 2008).
An increased supply of fatty acids due to excessive dietary intake of carbohydrates, leads to increased reactive oxygen species (ROS), like superoxide or hydrogen peroxide. ROS are generated during the oxidization of fat to carbon dioxide and water. Impaired antioxidant defense mechanisms might favor the accumulation of ROS, as hepatic lipotoxicity also leads to formation of ROS due to abnormal fatty acid (FA) oxidation. Also, cellular membrane FA and phospholipid compositions, as well as cholesterol content and ceramide/lipid signaling are altered. These include impairment of mitochondrial function or formation of cholesterol crystals, leading to hepatocyte degradation and activation of Kupffer cells (Tirosh, 2018; Ioannou et al., 2019). Both, a high fat diet or inflammatory environment can stimulate ceramide synthesis, which in turn regulates pro-inflammatory gene expression through the activation of NF-κB, leading to a positive feedback loop (Frangioudakis et al., 2010; Pagadala et al., 2012).
3. Receptor interacting serine/threonine kinases (RIPK) and caspases in steatotic and lean liver
The increased sensitivity of steatotic livers to cell death especially in the setting of IRI, represents a major clinical problem during liver transplantation. The underlying molecular mechanisms are still poorly understood (Kolachala et al., 2019; Sun et al., 2019).
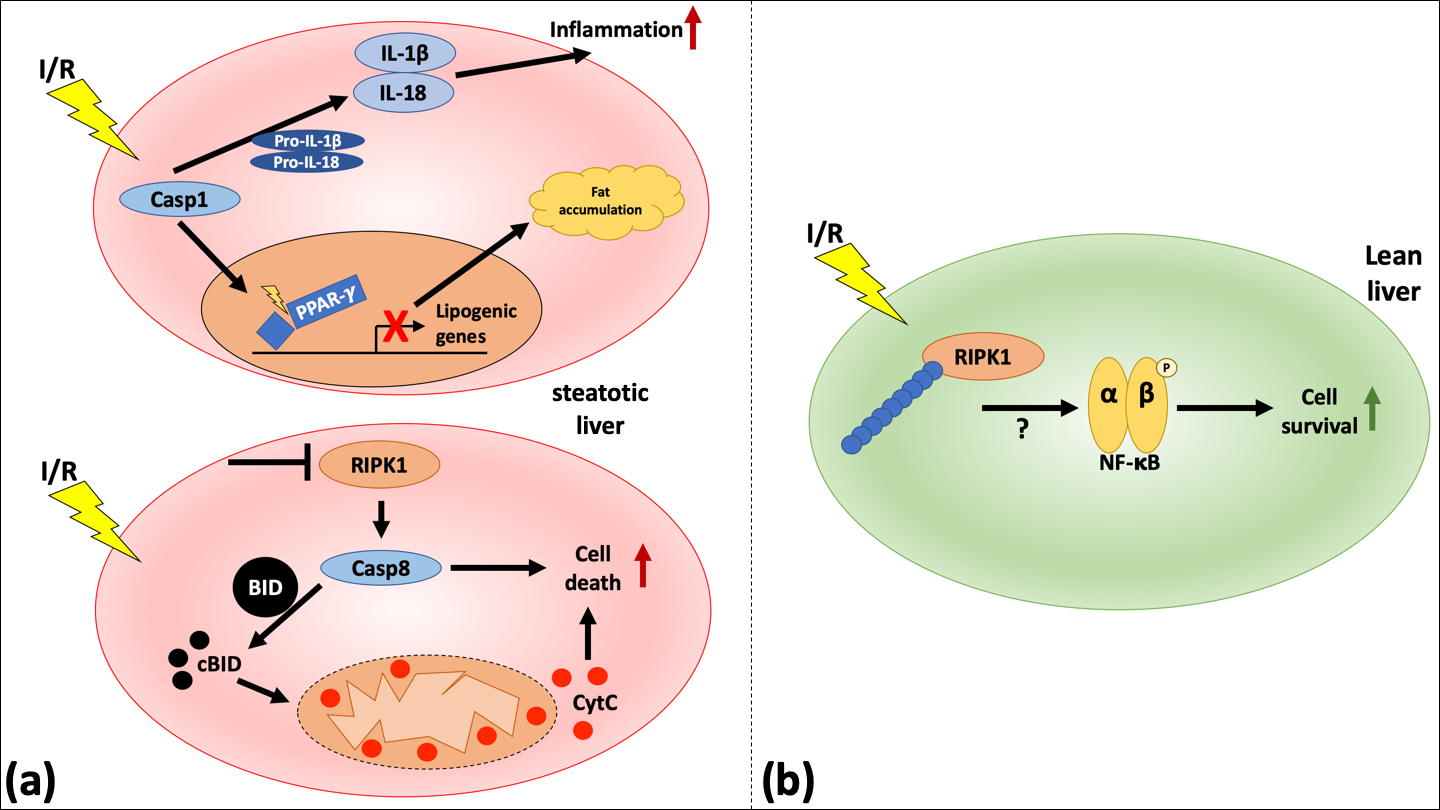
Hepatocyte cell death can be mediated by RIPK1- and RIPK3-dependent necroptosome (Newton, 2015; Wegner et al., 2017), caspase (Casp)1- and Casp11-dependent inflammasomes (Kanneganti et al., 2006; Martinon and Tschopp, 2007), and Casp8- and Casp9-dependent apoptotic pathways (Fig. 1) (Kim et al., 2000; Feldstein et al., 2003; Freimuth et al., 2013).
In a new window | Download PPT
Figure 1: Schematic representation of RIPK1-mediated signaling in steatotic compared to lean livers undergoing IRI. (a, upper panel) A priming signal leads to the activation of Casp1 and ultimately to the secretion of mature IL-1β and IL-18 and an inflammatory reaction. In another step, Casp1 can also lead to the cleavage of PPAR-γ, thereby influencing the metabolic pathway through downregulation of lipogenic genes, leading to fat accumulation. (a, lower panel) An increased hepatocellular injury in steatotic livers undergoing IRI through the inhibition of RIPK1 is mediated by Casp8. This pathway leads to apoptotic cell death and is only visible in steatotic livers. (b) In lean livers, the modification of RIPK1 by either K63 or linear chain ubiquitination leads to the activation of NF-κB and increasing cell survival.
RIPK1/RIPK3 signaling has been shown to mediate mostly pronecrotic functions (Cho et al., 2009; Vandenabeele et al., 2010). However, others demonstrated a prosurvival function, for example in the maintenance of the intestinal epithelial integrity of the gut (Dannappel et al., 2014; Takahashi et al., 2014). A study by Kolachala et al. (2019) demonstrates a difference between steatotic and lean livers on RIPK1-mediated cell death. The study suggests that in mice, RIPK1 inhibition in steatotic livers leads to increased Casp8-mediated cell death, whereas it protects lean livers. A mechanism by which Casp8 might mediate cellular death is through mitochondrial damage via the cleavage of the pro-apoptotic member of the B-cell lymphoma 2 (Bcl-2) family, BH3-interacting domain death agonist (BID). It was shown that a RIPK1 blockage in mice undergoing hepatic I/R after a high-fat diet led to increased mitochondrial damage, which was reversed in bid-/- mice (Kolachala et al., 2019). Interestingly, blockage of RIPK1 in lean mice attenuates IRI (Kolachala et al., 2019). Moreover, ubiquitination of RIPK1 in the steatotic liver was decreased compared to lean liver at baseline and after IRI. The study by Kolachala et al. (2019) also showed that in humans, steatotic livers have increased levels of Casp8, suggesting a translational significance. During the inflammatory response induced by damage-associated molecular patterns (DAMPs) and lipotoxicity, the NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome, as well as Casp1 are activated (Vandanmagsar et al., 2011). Moreover, different models of obese mice showed an activation of Casp1 (Stienstra et al., 2010; Koenen et al., 2011; Stienstra et al., 2011). Casp1 itself triggers an inflammatory response through the cleavage of the immature forms of interleukin (IL)-1βand IL-18, and influences metabolism by cleaving peroxisome proliferator-activated gamma (PPAR-γ), which potentially leads to a reduction of lipogenic genes, a disruption of metabolism, and hepatic fat accumulation (He et al., 2008; Guilherme et al., 2009; Keuper et al., 2013; Lamkanfi and Dixit, 2014).
Clinical approaches for the reconditioning of fatty livers
At the moment, it is estimated that by 2030, 86% of adults in the USA will be overweight or obese, which may ultimately lead to an increased incidence of NAFLD (Wang et al., 2008). Currently there are no approved drug treatments for NAFLD (Chalasani et al., 2018). However, there are drugs in ongoing phase 2 or phase 3 clinical trials (Sanyal et al., 2010; Neuschwander-Tetri et al., 2015; Armstrong et al., 2016; Ratziu et al., 2016; Friedman et al., 2018; Loomba et al., 2018; Linares et al., 2019). Some of these approaches will be presented in the following sections.
1. PPAR agonists for the reduction of hepatic fat accumulation
There are three PPAR isoforms, of which alpha (α) is largely present in the liver (Liss and Finck, 2017). There, it plays a role in regulating a variety of processes from fatty acid uptake and β-oxidation to inflammatory responses through the regulation of NF-κB (Desvergne and Wahli, 1999; Vandenabeele et al., 2003; Cave et al., 2016). Clinical data suggest that PPAR-α agonists may lower serum alanine aminotransferase (ALT) levels compared to a control group. In a pilot study, which included 16 NAFLD patients, lower plasma ALT concentrations were found after a treatment with fenofibrate, a PPARα agonist, for 48 weeks (Fernández-Miranda et al., 2008). A separate study showed that the PPARα agonist gemfibrozil also resulted in a lower ALT serum concentration (Basaranoglu et al., 1999). However, both studies failed to find any improvements in histological endpoints.
Although the isoform PPAR-σ is mostly found in skeletal muscle, it is also expressed by hepatocytes, as well as Kupffer cells and stellate cells, and might therefore be involved in hepatic inflammation (Tanaka et al., 2003; Tailleux et al., 2012; Cave et al., 2016). A study on overweight patients with dyslipidemia evaluated the PPAR-σ agonist MBX-8025. The study found significant reductions in apolipoprotein B-100, low density lipoprotein (LDL), triglycerides, non-high-density lipoprotein cholesterol, free fatty acids, and high-sensitivity C-reactive protein, as well as gamma-glutamyl transferase (GGT). However, other markers of liver injury have not been measured (Bays et al., 2011). Considering the role of PPAR-σ in fatty acid oxidation in skeletal muscles, the improved lipid profile might be connected to an overall increased energy consumption. In rodents it was shown that PPAR-σ agonists increase the oxygen consumption rates (Tanaka et al., 2003).
Although PPAR-γ is mostly expressed in adipose tissue, its increased expression in hepatocytes was shown in NAFLD patients (Nakamuta et al., 2005; Pettinelli and Videla, 2011). Thiazolidinediones (TZDs) are the most studied PPAR-γ agonists and ultimately enhance hepatic fatty acid oxidation (Maeda et al., 2001; Yamauchi et al., 2003; Brunt et al., 2015). One derivative of TZDs is rosiglitazone, which was tested in a study on NASH patients (Neuschwander-Tetri et al., 2003). After 48 weeks of treatment, ALT levels and steatosis, as well as hepatocyte ballooning decreased, resulting in 45% of patients not meeting the criteria of NASH anymore. However, six months after discontinuing, ALT levels increased again to pretreatment levels. Another PPAR-γ agonist is pioglitazone. NASH patients treated with pioglitazone for 12 months showed significant improvements in hepatocellular injury, as evaluated by hepatocyte ballooning, apoptosis, and necrosis compared to placebo (Aithal et al., 2008). There was, however, no improvement in steatosis or inflammation.
In another study, pioglitazone was compared to a treatment with the antioxidant vitamin E in 247 NASH patients, receiving treatment for 96 weeks (Sanyal et al., 2010). Pioglitazone led to a decrease in serum ALT and aspartate aminotransferase (AST) levels, hepatic steatosis, and lobular inflammation. However, histological features did not improve and patients gained more weight following pioglitazone treatment compared to placebo, which was previously also confirmed by others. Interestingly, the subgroup, which received vitamin E treatment did show an improvement in histological features and an improvement in nonalcoholic steatohepatitis after receiving 800 IU daily for 96 weeks. In this study vitamin E also reduced serum ALT and AST levels, as well as hepatic steatosis and lobular inflammation. In rodent NASH models, vitamin E also led to a reduction in fibrosis and a downregulation of inflammatory genes (Nan et al., 2009). Despite the potential protective effect of different substances in NAFLD patients, the de-fattening characteristics of these substances could potentially also be used for the reconditioning of marginal organs prior to implantation in machine perfusion to improve the outcome of patients.
2. Machine perfusion techniques to recondition fatty livers
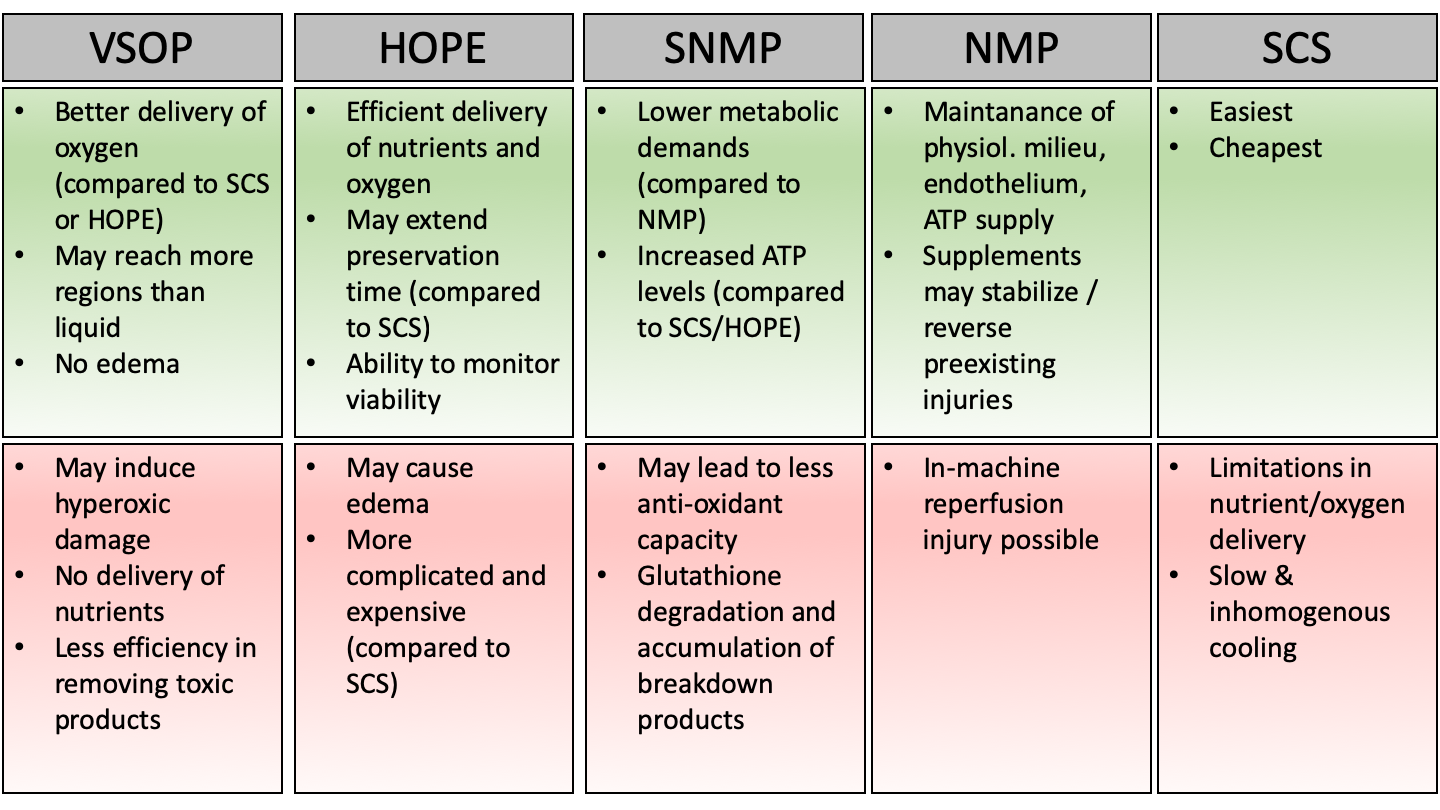
Today, different methods of machine perfusion exist and represent the most promising tool in protecting fatty livers before transplantation (Fig. 2). Through machine perfusion, it is possible to apply organ specific substances in order to modulate organ viability. Moreover, the ischemic time could be decreased through oxygenation of the grafts.
In a new window | Download PPT
Figure 2: Advantages (green) and disadvantages (red) of different machine perfusion techniques.
Venous systemic oxygenated persufflation (VSOP)
VSOP is performed by insufflation of gaseous oxygen into the hepatic vein before and during the cold storage time (Linares et al., 2019). The gas subsequently escapes through multiple small holes on the surface of the organ. Several preclinical studies investigated the effect of VSOP on liver IRI in different models. In 2009, Minor et al. (2009) studied the use of persufflation for the preservation of fatty livers. Livers of rats with macrovesicular steatosis underwent 20 h of SCS. One liver additionally underwent hypothermic reconditioning during the last 90 min of storage by insufflation of gaseous oxygen into the caval vein. Persufflation in this case led to decreased hepatic lipid peroxidation, cellular apoptosis, and autophagy. Moreover, the microscopic morphology, as well as the metabolic status of these livers, was improved.
Another study found that persufflation of fatty livers with gaseous oxygen and nitric oxide led to increased microcirculation and portal venous flow compared to SCS (Nagai et al., 2013). Also, serum ALT and interleukin-6 (IL-6) levels were lower after transplantation of the fatty liver. However, the group did not compare the results to a group of persufflation without nitric oxide. Taken together, preclinical studies in rodents showed that VSOP results in decreased parenchyma and mitochondrial damage and seems to have a positive effect on inflammation, as assessed by decreased IL-6 levels and attenuated postischemic Kupffer cell activation (Ye et al., 2010; Nagai et al., 2013). VSOP might also improve grafts microcirculation and lead to a reduction in necrosis, as assessed by histology, following hematoxylin and eosin staining (Minor et al., 2000; Minor et al., 2009).
Treckmann et al. (2008) showed in a pilot study with five patients the benefit of VSOP. Persufflation was applied to marginal liver grafts with less than 20% steatosis, which were previously rejected by three transplant centers due to warm ischemic damage prior to explantation. All recipients survived for the minimum follow-up of two years and did not need retransplantation. Another more recent study from 2019 enrolled 116 patients who either received a graft after 2 h of oxygen persufflation or after SCS (Gallinat et al., 2019). Of note, included were only livers from donors over 55 years old that were marginal organs reject multiple times by other centers. Tumor necrosis factor-α (TNF-α) serum levels were significantly reduced after oxygen persufflation. A subgroup analysis revealed a beneficial effect of oxygen persufflation concerning a development of EAD in donors with a history of cardiopulmonary resuscitation and elevated ALT, as well as concerning older and macrosteatotic livers. However, the study failed to show a difference between both groups in its main outcome parameters, median peak-AST levels, five-year graft, and patient survival (Gallinat et al., 2019).
Hypothermic oxygenated machine perfusion (HOPE)
Another dynamic preservation strategy for the use of marginal organs is HOPE. So far, HOPE has shown strong protective effects in various animal and human studies in terms of graft function after extended warm ischemia (Schlegel et al., 2013; Dutkowski et al., 2014; Schlegel et al., 2014; Dutkowski et al., 2015). Interestingly, several preclinical studies also suggest a positive effect of HOPE on fatty liver grafts. When comparing livers after 24 h of either SCS or HOPE in a rat model, livers after SCS showed significantly more damage, while bile production, ammonia clearance, as well as oxygen consumption and ATP levels were higher after HOPE (Bessems et al., 2007). However, these results were obtained ex vivo and need to be confirmed by a model of liver transplantation with end points like animal and graft survival. Kron et al. (2017) showed in a recent study the influence of HOPE in a rat model of fatty liver transplantation. HOPE treatment after cold storage of grafts was not able to decrease steatosis itself. However, the degree of reperfusion injury was markedly attenuated. They showed a significant reduction in mitochondrial oxidative stress, as assessed by oxidized DNA (8-hydroxy-2-deoxy guanosine), as well as a decrease in DAMP release (high mobility group box-1 protein), endothelial cell activation (Von Willebrand factor, endothelin-1, intercellular-adhesion-molecule-1) and Kupffer cell activation (Toll-like receptor- 4, myeloid differentiation primary response-88 induction), compared to SCS.
In a preliminary clinical study, six steatotic livers (20-40% macrosteatosis, 20-90% microsteatosis) were transplanted following HOPE treatment (Kron et al., 2017). Following transplantation, all grafts showed regular function, with a low peak in AST, without the development of PNF or retransplantation. Importantly, patients showed a reduced intensive care unit (ICU) length of stay (three days median ICU stay) and only one patient needed hemodialysis. All patients survived for one year. The group was compared to 12 recipients of un-perfused macrosteatotic livers (25-60% macrosteatosis, 10-80% microsteatosis), which were matched for donor and recipient age and total preservation time. Three of 12 recipients showed PNF, and three needed retransplantation. Moreover, 75% needed hemodialysis and the mean peak AST level was double that of the HOPE group. The ICU stay was 15 days and the one-year-survival rate was 42%.
The results of a phase 2 clinical trial (NCT01317342), which tests the influence of one hour of HOPE prior to liver transplantation on 70 participants, are still pending. In general, hypothermic machine perfusion seems to improve fatty liver preservation, compared to cold storage (Bessems et al., 2007). However, the true benefit of one technique over the other remains unclear, as studies directly comparing both show conflicting results (Guarrera et al., 2010; Henry et al., 2012; Suszynski et al., 2012; Bellini et al., 2019).
Subnormothermic machine perfusion (SNMP)
Vairetti et al. (2009) developed machine perfusion at subnormothermic temperatures (20 °C). They compared the influence of SNMP on fatty and lean rat livers and compared these results to machine perfusion at 4 °C and 8 °C. They found that machine perfusion at low temperatures is able to decrease fatty liver necrosis, compared to SCS. However, SNMP further protected fatty livers. Interestingly, there was no difference in necrosis in lean livers between the procedures. Upon further analysis, they found that steatotic livers preserved by machine perfusion at 20 °C showed a higher ATP/ADP ratio and lower oxidative stress, compared to SCS, indicating less mitochondrial dysfunction. Moreover, steatotic liver preserved by SCS showed an increased inflammatory response, compared to preservation by machine perfusion at 20 °C, as assessed by TNF-α and Casp3. Consequently, in a rat model SNMP also leads to less apoptosis of hepatocytes, as well as sinusoidal cells and an overall preserved hepatic ultrastructure, when compared to SCS (Boncompagni et al., 2011).
When considering steatotic livers for transplantation, a potential de-fattening option through machine perfusion needs to be considered. Liu et al. (2013) compared rat steatotic livers, which were perfused for six hours with either a de-fattening cocktail or a control substance. Although they were able to show a lipid export through SNMP, there was no significant difference between the de-fattening perfusate and the control substance.
They speculated that the lipid export during SNMP is slower, compared to normothermic machine perfusion and a potential benefit from a de-fattening cocktail in SNMP might only show at extended duration of perfusion.
To our knowledge, there is currently no clinical study using SNMP being conducted. Although the decrease in metabolism in SNMP might be favorable in terms of oxygen, for example, which still allows oxygenation through diffusion, the sustained metabolism in normothermic machine perfusion might be beneficial in de-fattening marginal organs.
Normothermic machine perfusion (NMP)
During NMP, the donor organ is preserved at body temperature and provided with oxygen and nutrients during transportation until transplantation. Because metabolism is still working, a pharmacological intervention is possible during perfusion and might be used to increase the usage of marginal organs by de-fattening, for example. In animal models, it has already been shown that de-fattening cocktails administered by NMP can be superior compared to control substances. For example, cultured fatty hepatocytes showed an increased expression of triacylglycerol hydrolase when cultured in medium containing a de-fattening cocktail, compared to a control culture medium, leading to increased export and oxidation of triacylglycerols (Nagrath et al., 2009). In this context, the PPAR isoforms have previously been shown to be involved in β-oxidation pathways and are also increased in hepatocytes cultured in de-fattening cocktails. In a next step, the most effective de-fattening cocktail was used in ex vivo experiments using steatotic rat livers and led to a significant reduction in intracellular lipid content as compared to a dimethyl sulfoxide control substance (Nagrath et al., 2009).
In another study Casp8 siRNA was administered one hour before ischemia in an in vivo mouse model of I/R (Contreras et al., 2004). For survival analysis, mice were subjected to 45 min. of total ischemia. Interestingly, while all control mice died within a five-day-period following I/R, 30% of mice, which received Casp8 siRNA survived for over 30 days. Delivery of Casp8 siRNA during machine perfusion might be another possible way to influence the apoptotic pathway. Indeed, in a phase 2 clinical trial on cold ischemia/warm reperfusion during liver transplantation, it was shown that a pan-caspase inhibitor can reduce serum transaminase concentrations in the recipient (Baskin-Bey et al., 2007). Interestingly, liver injury was only reduced, when the organ was flushed with the pan-caspase inhibitor. A group, in which additionally organ recipients received the drug, showed no beneficial effects, compared to the placebo control group. A possible explanation could be influence from the immune system of the recipients. For example, it could be hypothesized that caspase inhibitors can augment the number of neutrophils by decreasing apoptosis, thereby contributing to an extended inflammatory response (Khreiss et al., 2004; Perianayagam et al., 2004).
However, research on human liver in this field is usually limited to livers, which were previously rejected for transplantation and therefore are of fairly unpredictable nature. During a preliminary study including seven discarded human livers, two livers were circulated using a de-fattening protocol (Banan et al., 2016). While both livers did not show any I/R-associated pathological changes, only one showed a mild decrease in macroglobular steatosis of 10%. Another study demonstrated the overall feasibility of NMP on human donor livers (Akateh et al., 2018). In this study, four rejected human livers were first subjected to 5-9 h of cold storage, before undergoing 6 h of NMP. Sampling before and after perfusion confirmed a preserved morphology and no additional hepatocellular damage (Op den Dries et al., 2013).
The first randomized clinical trial on NMP was published in 2018 and included 121 NMP and 101 SCS cases (Nasralla et al., 2018). The authors showed a significantly lower discard rate and lower peak AST levels after transplantation of livers from circulatory death donors. Moreover, the rate of early allograft dysfunction was substantially decreased in the NMP group, compared to the SCS group. Also in 2018, another group published the first case of ischemia-free organ transplantation, in which a steatotic liver graft (85-95% macrosteatosis) was preserved under continuous NMP throughout the procedure (He et al., 2018). Interestingly, the recipient recovered with a functioning liver graft and showed minimal organ injury, as well as minimal inflammation. Taken together, NMP preservation seems to be superior compared to SCS. The main reasons may result from maintenance of liver metabolism, allowing for manipulation of grafts prior to surgery. NMP also influences the inflammatory reaction, for example by reducing the numbers of cluster of differentiation (CD)4- and CD8-positive T cells, thereby decreasing interferon (IFN)-γ and IL-17 (Jassem et al., 2019). In this context, Jassem et al. (2019) showed in a small study, which included 39 liver transplantations, that after NMP, liver grafts showed a 2.3-fold increase in regulatory T cells (Tregs), compared to SCS. It is thought that Tregs are able to decrease IRI.
Conclusion
Due to the longstanding discrepancy between the numbers of recipients on waiting lists and numbers of donor organs, many approaches are being developed to make more organs available. It is likely that different preservation techniques could not only lengthen the allowed ischemic time between ex- and implantation, but might also be used to recondition unfavorable organs. Today, the only treatment option for NASH is weight loss. However, given the growing incidence of liver steatosis, solid preservation strategies are crucially needed. Further understanding about the influence of the overall graft conditioning and the suitability of different machine perfusion protocols is needed and has the potential to increase the donor pool and close the gap between supply and demand.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This study was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft; EM 307/1-1 to C.E.; 2019), NIH R01 DK121330 (to C.J.), and STO 1099/8-1 to C.S.).
References
Christoph Emontzpohl1
1Department of Anesthesiology, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
Steve Bynon2
2Department of Surgery, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
Christian Stoppe3
3Department of Intensive and Intermediate Care, University Hospital Aachen, Aachen, Germany.
Gernot Marx3
3Department of Intensive and Intermediate Care, University Hospital Aachen, Aachen, Germany.
Cynthia Ju1
1Department of Anesthesiology, The University of Texas Health Science Center at Houston, McGovern Medical School, Houston, TX, USA.
Corresponding author:
Christoph Emontzpohl
Email: Christoph.Emontzpohl@uth.tmc.edu

In a new window | Download PPT
Figure 1: Schematic representation of RIPK1-mediated signaling in steatotic compared to lean livers undergoing IRI. (a, upper panel) A priming signal leads to the activation of Casp1 and ultimately to the secretion of mature IL-1β and IL-18 and an inflammatory reaction. In another step, Casp1 can also lead to the cleavage of PPAR-γ, thereby influencing the metabolic pathway through downregulation of lipogenic genes, leading to fat accumulation. (a, lower panel) An increased hepatocellular injury in steatotic livers undergoing IRI through the inhibition of RIPK1 is mediated by Casp8. This pathway leads to apoptotic cell death and is only visible in steatotic livers. (b) In lean livers, the modification of RIPK1 by either K63 or linear chain ubiquitination leads to the activation of NF-κB and increasing cell survival.

In a new window | Download PPT
Figure 2: Advantages (green) and disadvantages (red) of different machine perfusion techniques.
Supporting Information
Metrics
| Full-Text | Supporting Information | ||
|---|---|---|---|
| Number | 8910 | 14 | 0 |
Copyright © 2017 Conditioning Medicine, All Rights Reserved.
Address: Conditioning Medicine Editorial Office, 3500 Terrace Street, Pittsburgh, PA, 15213, USA